3878
Distortion-free, radial under-sampled fMRI with GRASP reconstruction1Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Golden-angle RAdial Sparse Parallel MRI (GRASP) is a fast field echo radial acquisition with golden-angle rotation sequence used in dynamic imaging due to its motion robustness and high temporal and spatial resolution. In this study, we implemented GRASP for BOLD fMRI during visual stimulation, which yielded distortion-free images in addition to reliable activation maps and signal time-courses as compared to EPI-based fMRI. This initial feasibility study suggests that GRASP could be extended towards other parts of the brain to serve as an alternative when EPI suffers from signal distortion.
Introduction
Golden-angle RAdial Sparse Parallel MRI (GRASP) is an MRI technique that combines compressed sensing, parallel imaging, and golden-angle radial sampling. GRASP has demonstrated motion robustness and high temporal and spatial resolution in dynamic imaging, and has been one of the most notable advances in the MR field in the past few years1. Echo Planar Imaging (EPI), which is the conventional acquisition method of fMRI, may suffer from motion artifacts and spatial distortion at air and brain tissue interfaces2. The goal of this study was to demonstrate the initial feasibility of using GRASP for BOLD fMRI.Methods
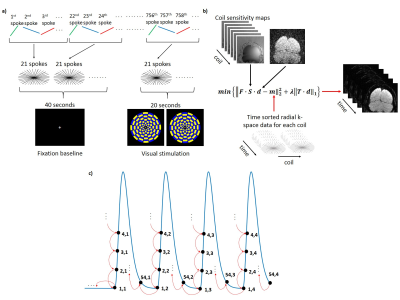
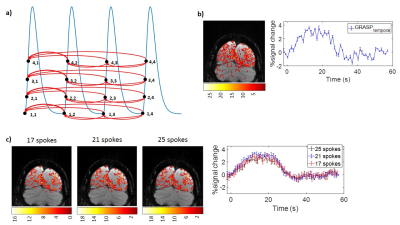
MRI data acquisition3T MRI scans were acquired in two healthy controls (1F/1M, 22 yrs). A visual fMRI paradigm was used, consisting of an initial 40 second black screen as baseline, followed by 4x 20 second flashing checkerboards and 40 second black screen. The fMRI scan using 2D fast-field-echo radial acquisition with golden-angle rotation sequence employed the following parameters (Figure 1a): FOV: 140x140x3mm3, TR/TE: 52.95/25ms, 5292 spokes, voxel size: 0.547mm, total scan time: 280 seconds. An oblique slice intersecting the calcarine fissure was used and the slice position is shown in Figure 2a. All acquired spokes were divided into 251 dynamics, with 21 spokes in each dynamic and a TR of 1.1 seconds. For comparison, an EPI-based fMRI was also performed using identical scan time and voxel size.
GRASP reconstruction
A flow chart of the GRASP reconstruction method is shown in Figure 1b. Radial k-space data for each coil were re-sorted into 21 consecutive spokes/frame. The inverse non-uniform fast Fourier transform (NUFFT) reconstructed image was used as an initial input with the goal of minimizing the difference between the NUFFT image and k-space data (data consistency). Additionally, a temporal total-variation operator (Figure 1c) was used on the l1 norm to minimize differences between adjacent frames (sparsity constraint)1. This temporal constraint was placed because 1) BOLD signal is relatively slow-changing; 2) only a small portion of the brain is expected to be activated, thus the majority of the voxels in the image should be temporally unchanged. To investigate the effect of varying parameters in the GRASP reconstruction method, we used a range of weighting parameters, ƛ=0-0.5 (Figure 2b) which controls the degree of tradeoff between data consistency and sparsity constraint.
fMRI processing
Activation maps (thresholded at p=0.05 and a cluster size of 20 voxels) associated with visual stimulation were obtained from a general linear model analysis. An ROI of the primary visual cortex was manually drawn and activated voxels inside the ROI were used for quantitative BOLD time-course analysis.
Additional testing of the GRASP reconstruction method
We also implemented a cyclic total-variation operator that constrains each time point in a cycle to its counterpart time point across all previous cycles (Figure 4a). This constraint is based on the notion that the same time points in different stimulus cycles should be minimally different.
In addition, we tested the effect of spoke number per image on the final results. We compared 17, 21 and 25 spokes/frame, corresponding to temporal resolutions of 0.9, 1.1, and 1.3 seconds in the fMRI data.
Results and Discussion
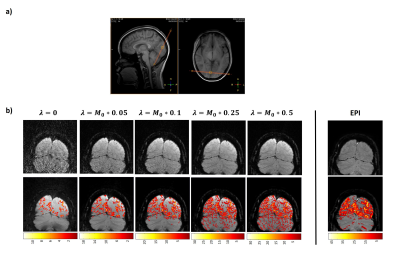
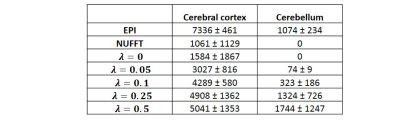
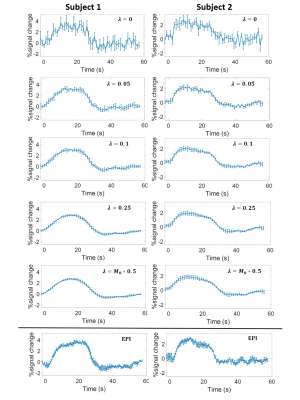
Figure 2b (top row) shows a representative comparison of GRASP and EPI BOLD images of a single dynamic from one subject. The GRASP images show a reduced amount of aliasing artifacts as the temporal constraint factor, ƛ increases. Note the absence of distortion in GRASP images. The activation maps (Figure 2b, bottom row) show an increase in activated voxels in the visual cortex with ƛ (Table 1). On the other hand, spurious activations in unrelated regions (e.g. cerebellum) are also seen as the temporal constraint factor, ƛ became greater (Table 1). It appears that a ƛ of 0.05 yielded the best tradeoff between sensitivity and specificity in GRASP fMRI. EPI fMRI revealed a higher number of activated voxels but also showed sizable false-positives in the cerebellum (Figure 2b and Table 1).Figure 3 shows BOLD time-courses averaged across four cycles (shown for both subjects). It can be seen that, as ƛ increases, the hemodynamic curve becomes increasingly reliable with smaller inter-cycle error bars. However, there is also a smoothing effect. A ƛ of 0.05 yields a curve comparable to that of EPI BOLD (bottom of Figure 3).
Figure 4a shows the constraint scheme based on minimal inter-cycle signal differences. Figure 4b shows the activation map and the corresponding time-course. It can be seen that the time-course is noisy but the inter-cycle error bars are artificially small (because of the reconstruction constraints).
Finally, we tested the impact of the number of spokes in each frame, by using different spoke number of 19, 21, and 25. Figure 4c shows activation maps and BOLD time-course as a function of spoke number, which yielded similar results.
Conclusion
This is the first report to use a novel radial-acquisition technique for fMRI brain mapping that demonstrated reliable activation maps and signal time-courses. Although this initial feasibility study was performed in the visual cortex, GRASP could be extended to other parts of the brain where EPI is known to be prone to signal distortion.Acknowledgements
No acknowledgement found.References
1. Feng, L., Grimm, R., Block, K.T., Chandarana, H., Kim, S., Xu, J., Axel, L., Sodickson, D.K. & Otazo, R. Golden‐angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden‐angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med 2014; 72:707-717.
2. Glover, G.H. Overview of Functional Magnetic Resonance Imaging. Neurosurgery Clinics of Nother America 2011; 22:133-139.
Figures