3872
Effect of Coil Compression on fMRI Time Courses: Preliminary Analysis1Applications and Workflow, GE Healthcare, Buc, France, 2Applications and Workflow, GE Healthcare, Waukesha, WI, United States, 3Engineering, GE Healthcare, Waukesha, WI, United States, 4Applications and Workflow, GE Healthcare, Menlo Park, CA, United States
Synopsis
Coil compression was introduced as a mean to reduce image reconstruction computational complexity of data acquired with large coil arrays with negligible SNR penalty. Previous work using a different acquisition strategy (not a standard multiband EPI) suggests that coil compression has a negligible effect on fMRI time courses. In this preliminary study, the effects of our coil compression implementation on fMRI time courses is evaluated using different level of compression and a standard multiband EPI. The results suggest a negligible effects of coil compression on fMRI time courses.
INTRODUCTION
The coil compression (CC) approach was introduced as a mean to reduce image reconstruction computational complexity of data acquired with large coil arrays1. Chu et al. and others demonstrated that it has a negligible SNR penalty1-3. Recent advances in Multiband (MB) echo-planar imaging (EPI) allow to significantly increase the spatiotemporal resolution of fMRI4. This allows whole brain acquisitions at 2mm isotropic in 0.7-0.8s like in the human connectome project (HCP). However, MB-EPI requires large coils array to separate the slices. Consequently, the amount of data to reconstruct per unit time becomes quickly difficult to manage. A solution to reduce the computational complexity is to use CC with MB-EPI. In the preliminary study, the effect of coil compression on fMRI time courses is evaluated on both task and resting-state experiments.METHODS
Three volunteers underwent a MR exam using a 3.0T scanner (Signa Premier, GE Healthcare) using a 48-channels head-coil (GE Healthcare). The acquisition protocol included a 3D-T1-weighted (1mm isotropic), a resting-state fMRI and 3 finger-tapping tasks (left finger-tapping for volunteer 1, right finger-tapping for the others). The resting-state fMRI lasted 5:12 minutes and each finger-tapping task 3:00 minutes (block-design, 30s rest, 30s tapping, repeating 3 times). The acquisition parameters for fMRI were like the HCP protocol (2mm isotropic, FOV=208mm, matrix=104x104, thickness=2mm, TE=30ms, TR=0.8s, #slices=72). The fMRI raw data were saved for off-line reconstruction using different level of an eigen-value decomposition-based coil compression3: 1) without coil compression (referred as NoCC, the gold standard), 2) with the number of channels reduced to 32 (CC32) and 3) with the number of channels reduced to 24 (CC24).The reconstruction lag (time between “end-of-acquisition” and “end-of-reconstruction”) will be evaluated as a function of CC level.
The data pre-processing was performed using SPM12 and Matlab. The EPI data were realigned, corrected for slice-timing, co-registered to 3D-T1w, normalized to the MNI space, detrended (2nd order polynomial) and masked. The task data were finally smoothed at 4x4x4mm. For resting-state, data were bandpass-filtered ([0.008-0.1]Hz) and residualized (cleaned-up) using GLM with aCompCor5, motion parameters and their time-derivatives as nuisances (also bandpass-filtered as recommended6,7) and smoothed at 6x6x6mm. The task analysis was performed using a GLM to extract the task-response for NoCC, CC32 and CC24 for the three runs of finger-tapping per subject. For the resting-state, a seed-based analysis using a 5mm-radius sphere located in the posterior cingulate cortex (PCC, MNI coordinate [2 -54 26]) to extract the default mode network (DMN). ICA was also used (FSL MELODIC) but only the DMN was kept. In all cases, voxelwise familywise-error correction (FWE) was used (<0.05).
Additionally, a fBIRN phantom scan was performed for tSNR measurements (same protocol).
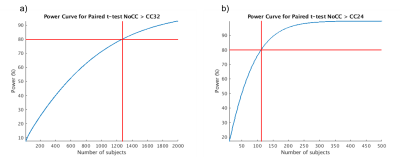
Finally, using the task data we estimated the number of subjects needed to detect the effect of CC in paired t-test NoCC > CC32 and NoCC > CC24 with 80% statistical power (with type I error of 5%) in a volume-of-interest corresponding to a right finger tapping (Postcentral_L taken from AAL2 atlas). This was done using fMRIpower tool8,9. To artificially increase the number of subjects for the power calculation the normalized EPI data of the first volunteer (left finger-tapping task) were left-right inverted, and all data were smoothed at 8x8x8mm to reduce potential effects of this uncommon procedure.
RESULTS
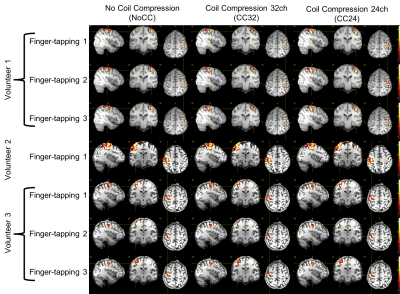
The reconstruction lag on the same hardware was 407/52/7 seconds for NoCC/CC32/CC24, respectively. This demonstrates the effectiveness of CC to reduce computational complexity.The 2nd and 3rd finger-tapping of volunteer 2 was excluded due to excessive motion. Task analysis results do not indicate any major difference between NoCC, CC32 and CC24: only extremely minor differences are noticeable (see figure 1).
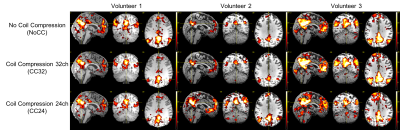
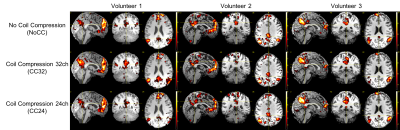
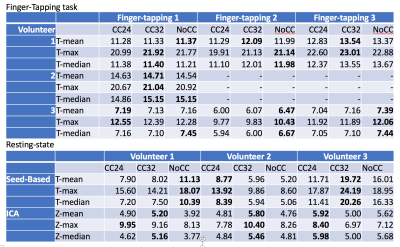
For the seed-based and ICA analyses (figure 2 and 3, respectively), the DMN were extracted successfully in all cases. We noticed some minor differences but no clear tendency between NoCC, CC32 and CC24. In figure 4, T- and Z-statistics are given. Again, this is no clear tendency that could help us to identify a good or bad effect of CC.
The fBIRN phantom tSNR measurements were 58.14±29.89 (NoCC), 58.18±29.10 (CC32) and 58.55±29.20 (CC24).
The power analysis (see figure 5) shows that we would need 1271 subjects to detect a difference for NoCC>CC32 and 112 subjects for NoCC>CC24 for a right finger-tapping task.
DISCUSSION
In this preliminary study, the task data shows only extremely small differences.For the resting state, we noticed stronger differences but no clear tendency between NoCC, CC32 and CC24. It is not impossible that theses minor differences in resting-state data are introduced by the clean-up procedure as the CompCor regressors are derived from the data, and this is the only stage where it could differ.
Additionally, the tSNR measurements on phantom did not reveal any effects of CC.
For the power analysis, the drop-in number of subjects from 1271 for NoCC>CC32 to 112 subjects for NoCC>CC24 might indicate that we should not push the compression level to a higher level.
CONCLUSION
These preliminary data suggest that coil compression with a reasonable level of compression can be used safely to reduce the computational complexity for high spatiotemporal resolution fMRI.Acknowledgements
No acknowledgement found.References
1. Chu A, Noll DC, Coil compression in simultaneous multislice functional MRI with concentric ring slice-GRAPPA and SENSE. Magn Reson Med, 2016, 76, 1196-1209.
2. Huang F, Vijayakumar S, Li Y, Hertel S, Duensing GR, A software channel compression technique for faster reconstruction with many channels. Magn Reson Imaging, 2008, 26, 133-141.
3. Zhang T, Pauly JM, Vasanawala SS, Lustig M. Coil compression for accelerated imaging with Cartesian sampling. Magn Reson Med, 2013, 69, 571-582
4. Uğurbil K, Xu J, Auerbach EJ, et al. Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project. Neuroimage, 2013, 80, 80-104.
5. Behzadi Y, Restom K, et al. A component-based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 2007, 37, 90-101.
6. Bright M, Tench C, et al. Potential pitfalls when denoising resting-state fMRI data using nuisance regression. NeuroImage, 2017, 154, 159-168.
7. Hallquist M, Hwang K, Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage, 2013, 82, 208-225.
8. Mumford JA, Nichols TE. Power calculation for group fMRI studies accounting for arbitrary design and temporal autocorrelation. NeuroImage, 2008, 39, 261-268.
9. Mumford JA. A power calculation guide for fMRI studies. Social cognitive and affective neuroscience, 2012, 7, 738-742.
Figures