3868
A novel motion correction for ASL-fMRI with multi-PLD: non-parametric Gaussian Processes prediction of background suppression1Institute of Biomedical Engineering, Department of Engineering Science, University of Oxford, Oxford, United Kingdom, 2Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 3Department of Radiology, University of California, La Jolla, CA, United States
Synopsis
For fMRI using ASL, multi-PLD acquisitions may have advantages, improving reliability and specificity. However, the varying static-tissue signal in multi-PLD ASL can confound motion estimation when conventional motion correction is applied. In this study, we propose a novel framework using Gaussian processes to address this problem, in which motionless ASL images are predicted, so that they can be used as a reference for motion correction for each ASL volume. Simulation and in-vivo studies show the new motion correction framework using Gaussian Processes eliminates the influence of multi-PLD and provides a suitable reference for each volume.
INTRODUCTION
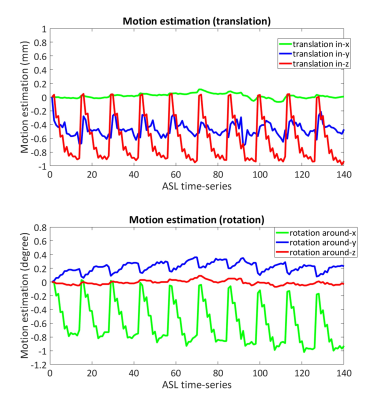
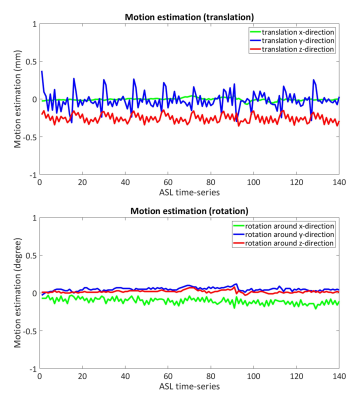
Cerebral blood flow (CBF) is an essential physiological parameter that shows a close coupling with neuronal activity. Whereas the Blood Oxygen Level Dependent (BOLD) technique is the traditional method for functional MRI (fMRI), several studies have shown the advantages of Arterial Spin Labeling (ASL), such as better spatial localization and CBF quantification1. For CBF quantification using ASL, while the acquisition with single post-labeling delay (PLD) is currently the most common approach, Mezue et.al. suggested that the use of multiple PLDs may improve the reliability and specificity by accounting for arterial transit time (ATT) variability1. However, when motion correction (MoCo) is required (which is especially important for ASL-fMRI to detect statistically significant activation from small regions of interest), choosing a suitable cost-function for multi-PLD ASL is difficult: In ASL, background (static-tissue signal) suppression (BGS) is performed to improve the SNR of CBF-map by suppressing physiological subtraction artefacts. However, it introduces problems for post-acquisition MoCo: The efficiency of BGS is often poorer for shorter PLD due to less flexibility for optimization, which means the static-tissue signal varies over different PLDs. Such varying tissue signal is not ideal for the use with the conventional image similarity measure for motion estimation, which results in poor motion estimates (Figure-1), and resulting artefacts following motion correction. In this study, we propose a novel MoCo framework for multi-PLD ASL data, in which motionless BGS-ASL images that present the identical static-tissue signal to each PLD is predicted by employing Gaussian Processes (GP)2, so that they can be used as a reference for MoCo for each ASL volume.METHODS
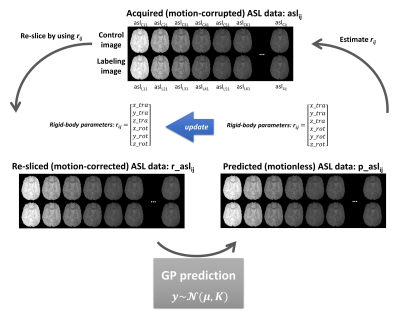
MoCo frameworkMotion estimation is performed using rigid-body registration between volumes in the ASL time-series. To minimize artefactual overestimation as shown in Figure-1, we aim to perform motion estimation on ‘ideal’ images of static-tissue having removed other sources of variation besides motion. Although it is theoretically possible to analytically predict the static-tissue signal after BGS if the underlying function and variables are known (e.g. T1-recovery function with T1-value, BGS-pulse timings, the efficiency of BGS and pre-saturation, as well as magnetization transfer effects caused by pCASL labelling), it is not practical to obtain all these parameters from the ASL time-series. Instead, we propose the use of GP as a non-parametric approach to predict the ideal static-tissue signal for each volume. Figure-2 explains the iteration process of the MoCo and prediction of BGS-ASL data. The acquired multi-PLD ASL volumes are used as the training dataset for GP. To minimize the influence of the subject’s motion in the training dataset, (i) the mean-function µ was modelled by using the mean value of the re-sliced ASL data for each PLD over entire epochs, and (ii) the length-scale of the covariance function K (squared exponential in this study) was set to 0.4 (larger than ΔPLD of 0.25s) to reflect that “smooth” variation in static tissue with PLD introduced by BGS. This cycle is repeated several times to obtain more accurate motion estimation.
In-vivo data acquisition and motion-simulation
We applied the framework to a previously acquired multi-PLD ASL-fMRI dataset1: single-shot echo-planar imaging pseudo-continuous ASL (pCASL) images with 6 PLDs (250, 500, 750, 1000, 1250 and 1500ms (ΔPLD of 250ms) after pCASL labelling of 1400ms) repeated 10 times (10 epochs) without any intentional head motion. Additionally, a BGS-ASL dataset was simulated by using the M0-image, CBF-map and T1-map and by analytically applying the signal attenuation caused by BGS and perfusion for each PLD. To help to observe the subtraction error, perfusion signal attenuation was applied only to the left hemisphere, so that all pixel values from the right hemisphere indicate the subtraction error. In 60 volumes of the simulated dataset (i.e. 5 epochs), rotation was applied around z-direction (yaw).
RESULTS
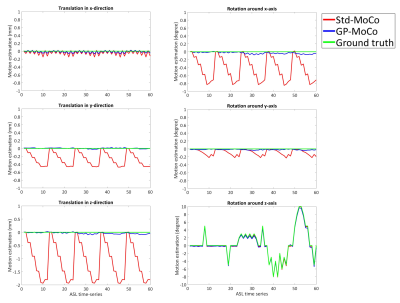
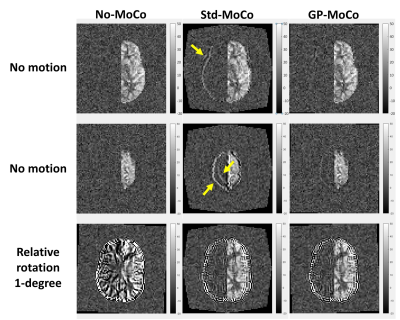
Figure-3 shows the estimated motion parameters in the simulation study. When applying a standard MoCo (Std-MoCo, using SPM12 in this study) to multi-PLD ASL, severe overestimation caused by varying static-tissue signal was observed, although the simulated motion (rotation around the z-axis) was correctly estimated. By applying our framework using GP (GP-MoCo), the motion estimation was very close to the ground truth. Figure-4 shows example images after control-label subtraction, in which Std-MoCo resulted in obvious subtraction errors as indicated by arrows. GP-MoCo avoided such subtraction errors, as well as successfully correct motion. Finally, Figure-5 shows that GP-MoCo successfully eliminated the wrong motion estimation of the in-vivo data shown in Figure-1.DISCUSSION AND CONCLUSION
This work aims to address the limitation of the use of multi-PLD ASL for fMRI studies; varying static-tissue signal over multiple PLDs hinders accurate motion estimation. Our new MoCo framework using GP provides the reference images presenting identical signal intensity to the BGS-ASL with multi-PLD, so that the motion estimation is not influenced by different BGS-efficiency over multiple PLDs. In this study, several iterations were required to reach an accurate motion estimation close to the ground truth. Potential future work includes further investigation for the selection of the covariance function and hyper-parameters that could improve the efficiency of results.Acknowledgements
This work was supported by Engineering and Physical Science Research Council (EP/P012361/1). The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z). TO is supported by the Royal Academy of Engineering.References
1. Mezue M, Segerdahl AR, Okell TW, Chappell MA, Kelly ME, Tracey I. Optimization and reliability of multiple postlabeling delay pseudo-continuous arterial spin labeling during rest and stimulus-induced functional task activation. J Cereb Blood Flow Metab. 2014; 34:1919-1927.
2. Rasmussen, CE and Williams, CKI. Gaussian processes for machine learning. MIT Press 2006.
Figures