3864
Beyond the limits of layer-dependent CBV fMRI in humans: strategies towards whole brain coverage, sub-second TR, and very high 0.5mm resolutions1MR-Methods group, MBIC, Faculty of Psychology and Neuroscience, Maastricht, Netherlands, 2SFIM, NIMH, Bethesda, MD, United States, 3German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 4Physics and Astronomy, University of Bonn, Bonn, Germany, 5UHN Toronto, Toronto, ON, Canada
Synopsis
Recent developments of ultra-high field (UHF) MRI and high-resolution CBV-sensitive VASO sequences have made it possible to measure activity changes across cortical depths. However, most methods are limited to individual brain areas of large cortical thicknesses (4mm in M1), long repetition times and small matrix sizes. In this study, we developed multiple VASO sequence approaches to achieve:
1.) Whole-brain coverage (104 slices) at sub-millimeter layer resolutions
2.) Sub-second TRs (TR = 650ms) at sub-millimeter layer resolutions
3.) Extra-high 0.5mm isotropic resolutions with matrix sizes of 316
We tested new sequence concepts and validated their applicability for the neuroscientific context.
Purpose
Layer-specific fMRI can address questions on directional feedforward and feedback neural information flow between brain areas [Felleman and VanEssen 1991]. However, the application of layer-fMRI is limited by multiple factors:- Venous leakage: Since the venous vessels are not as laminar aligned like the neurons are, conventional GE-BOLD fMRI contrasts are biased toward superficial layers [Polimeni 2010; Kay 2018].
- Low SNR: Non-BOLD fMRI contrasts, such as, the cerebral blood volume (CBV)-specific VASO contrast [Lu 2003] are thought to be locally more specific, while being less sensitive. The correspondingly low SNR does not allow spatial resolutions better than 0.8mm iso.
- Limited coverage:The original VASO sequence is inherently a single slice method. While low-resolution VASO could be extended to whole brain coverage at 3T [Poser 2011] and 7T [Hua 2013; Huber 2018], it has not been possible to achieve whole brain VASO coverage with sub-millimeter resolutions. This limitation of VASO comes from the fact that the duration of whole brain readouts would be longer than the duration of an inversion recovery time.
- Long TR: The long blood T1 at UHF results in a correspondingly long the blood-nulling time TI and TRs larger than 3sec.
Methods
2-15 min acquisition runs were conducted during a finger tapping task and a flickering checkerboard task, with N=10 participants. Sequence development in two participants was approved by Maastricht-FPN ethical review board: ERCPN:180_03_06_2017. 8 datasets were acquired under the NIH-IRB (93-M-0170, ClinicalTrials.gov: NCT00001360). Functional tasks consisted of 30s-30s rest-activation periods. Functional data of GE-BOLD and VASO were concurrently acquired with various versions of SS-SI-VASO [Huber 2014] (see Fig. 1). Common readout parameters were: TE=25ms, in-plane PF=6/8 with POCS#8, FLASH-GAPPA=3, 3D-EPI readout [Poser 2010; Stirnberg 2019], 7T (Siemens Healthineers).- Whole brain protocol: 0.8mm iso, 88-104 slices, (TR see Fig. 1)
- Sub-second TR protocol: 0.8mm iso, 8 slices, TR=650ms
- 0.5mm protocol: N=2 inplane segments, matrix 316, volume TR 4s.
- A) Long readout VASO: 3D-readout during single inversion-recovery relaxation
- B) Multi-slab VASO: Multi-slab VASO: Multiple thin 3D-slabs are acquired across a corresponding number of runs and concatenated post-hoc as previously done with high-res multi-slab ASL [Ivanov 2017]
- C) MAGEC VASO: (Multiple-Acquisition-with-Global-Excitation-Cycling): T1-weighting without inversion, which maintains the T1-contrast across k-space segments variable flip angles. Inspired by MAGIC VASO [Lu 2014] and VAPER [Chai 2018]
- D) TR-segmented VASO: 3D k-space acquisition across multiple inversion recovery periods. As implemented by [Stirnberg 2019]
- E) CAIPI VASO: GRAPPA acceleration along both phase encoding directions with controlled aliasing (shift=1) [Poser and Setsompop 2018]
Results
Fig. 2 shows the raw signal, the T1-weighted signal and tSNR maps of the VASO variants, tested here for the case of whole brain coverage at 0.8mm resolution. We find that all methods have tSNR values above 10. The method with the highest CNR efficiency ((tSNRA-tSNRB)/√TR) is the multi-slab approach (Fig. 1B). However, it can only be used in experiments with repeatable tasks. We obtain activation induced tapping activity with all whole brain VASO variants (examples shown in Fig. 3). Fig. 4 shows that the segmentation (Fig. 1D) approach can be used to achieve large matrix sizes and high isotropic resolution of 0.5mm. Since the MAGEC VASO approach does not rely on an inversion recovery delay (Fig. 1C), it can also be used to reduce the TR for fast CBV imaging (Fig. 5A).Discussion and Conclusion
Any layer-fMRI protocol (BOLD and non-BOLD) needs to find a compromise of a triangular parameter space: -TR, -coverage, and -spatial resolution (Fig. 5B). When one parameter is pushed to its limits, at least one other parameter needs to be traded off. In this study we developed new sequence approaches to flexibly move within this sequence parameter space. This is a large step towards making layer CBV-fMRI a practical and user friendly tool to measure directional information flow between brain areas, without the venous contamination of conventional GE-BOLD.The new approaches allow us to present data with unprecedented quality:
- first setup that allows researchers to collect whole brain layer-dependent VASO data. This is a paradigm shift for neuroscientific investigation of directional functional connectivity. (See examples of neuroscience applications with this whole-brain protocol here (Huber 2020, submitted to ISMRM: https://layerfmri.page.link/ISMRM20_movies).
- first setup that allows researchers to collect layer-fMRI VASO at sub-second TRs to reveal detailed differences in the VASO specific HRF compared to BOLD.
- we achieved the highest spatial resolution with VASO ever recorded.
Acknowledgements
- Laurentius Huber is funded by the NWO VENI project 016.Veni.198.032.
- Benedikt Poser and Deni Kurban received partial funding from 16.Vidi.178.052.
- Benedikt Poser and Sriranga Kashyap received funding from R01 MH111444/MH/NIMH NIH.
- Yuhui Chai, Peter Bandettini and Sean Marrett are funded from the Intramural Research Program of the National Institute of Mental Health (ZIAMH002783).
References
[Chai 2018] Chai et al., 2018, ISMRM, 6010, A Novel Intravascular Contrast for Laminar Functional MRI.
[Felleman and Van Essen 1991] Felleman and Van Essen, Cerebral Cortex, 1:1-113, Distributed hierarchical processing in the primate cerebral cortex.
[Hua 2014] Hua et al., 2013, MRM, 69:1003–1013. Mapping of arterial transit time by intravascular signal selection.
[Huber 2014] Huber et al., 2014, MRM 72:137-148, Slab-selective, BOLD-corrected VASO at 7 tesla provides measures of cerebral blood volume reactivity with high signal-to-noise ratio.
[Huber 2018] Huber, L. et al. Neuroimage 164:131–143, Techniques for blood volume fMRI with VASO: From low-resolution mapping towards sub-millimeter layer-dependent applications.
[Ivanov 2018] Ivanov et al., 2017, ISMRM, 2301, Human whole-brain sub-millimeter cerebral blood flow map using 7T ASL.
[Lu 2003] Lu et al., 2017, MRM, 50:63-74, Functional magnetic resonance imaging based on changes in vascular space occupancy.
[Polimeni 2010] Polimeni et al., 2010, NeuroImage, 53: 1334-1346, Laminar analysis of 7T BOLD using an imposed spatial activation pattern in human V1.
[Poser 2010] Poser et al., 2010, NeuroImage, 51: 261-266, Three dimensional echo-planar imaging at 7 tesla.
[Poser and Setsompop 2018] Poser, B. A. & Setsompop, K. Neuroimage 168:101–118. Pulse sequences and parallel imaging for high spatiotemporal resolution MRI at ultra-high field.
[Stirnberg 2019] Stirnberg et al., 2019: ISMRM 4420. T1 Mapping at 7T Using a Novel Inversion-Recovery Look-Locker 3D-EPI Sequence
Figures
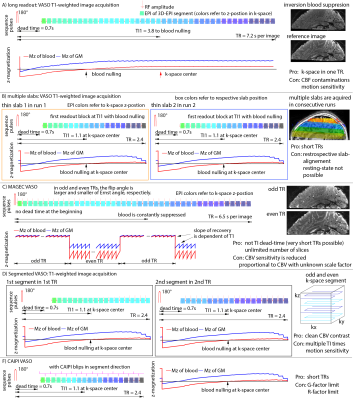
Fig. 1: Sequence strategies to achieve high-resolution, whole-brain for layer-dependent VASO fMRI.
All approaches achieve 88-104 slices with 0.8mm resolution. The T1-contrast mechanism and the acquisition timing is varied across methods. The individual rows refer to independent sequence strategies that can also be combined.
A high-resolution version of this figure is here: https://layerfmri.page.link/WholeBrain_ISMRM20
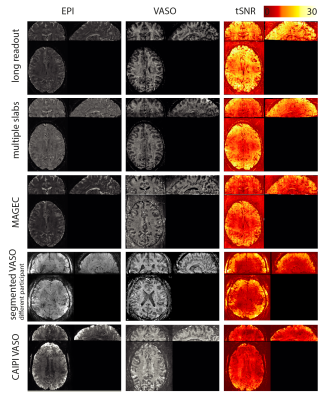
Fig. 2: Basic sequence quality measures across different whole brain VASO strategies.
tSNR values in GM are in the range of 12 (CAIPI VASO) up to 22 (long readout VASO). Due to the different TRs, different CBV weighting and the necessity to repeat experiments for multi-slab VASO, the contrast-to-noise (CNR) efficiency is not directly proportional to tSNR.
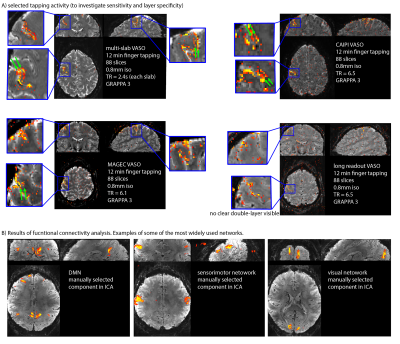
Fig. 4: Signal maps and functional results for MAGEC VASO, CAIPI VASO and multi-slab VASO.
It can be seen that the methods have enough sensitivity to detect significant activity changes at 0.8mm in 12min.
A) As expected from previous studies with small field of views (FOV) [Huber 2017], CBV changes are located in two stripes within the primary motor cortex (green arrows).
B) ICA evaluation in FSL-melodic show that the data-quality is sufficient to extract the common functional connectivity networks.
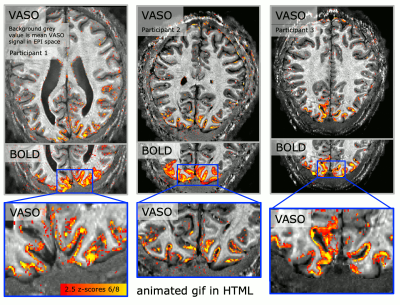
Fig. 4: 0.5 mm VASO results.
A matrix size of 316 was achievable by means of in-plane segmentation and GRAPPA acceleration of a factor of 6 (GRAPPA 3 x segmentation 2). In the animated gif (click on it to play it), it can be seen how the CBV-weighted VASO is more specific to the middle layer of the cortex. 28 slices are acquired.
High-resolution figure is avaliable here: https://layerfmri.page.link/WholeBrain_ISMRM20
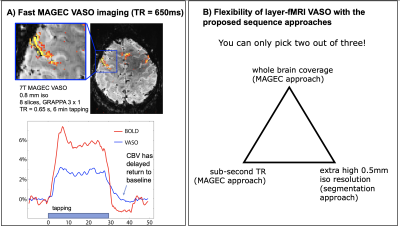
Fig. 5A MAGEC VASO for fast CBV mapping
The MAGEC VASO approach does not require an inversion recovery delay. Thus, the effective TR can be shorter than in conventional VASO approaches. Here, TR=650ms is doable by trading off the coverage size
Fig. 5B Conclusion figure
The proposed sequence approaches (specifically MAGEG and segmentation) provide more flexibility. As such, extra-high resolution, sub-second TR and whole brain coverage is achievable.