3863
Detection of Cortical Depth-dependent Functional Activation using Whole-brain, Half-millimetre Resolution EPIK at 7T
Seong Dae Yun1, Patricia Pais-Roldán1, and N. Jon Shah1,2,3,4
1Institute of Neuroscience and Medicine 4, INM-4, Forschungszentrum Juelich, Juelich, Germany, 2Institute of Neuroscience and Medicine 11, INM-11, Forschungszentrum Juelich, Juelich, Germany, 3JARA - BRAIN - Translational Medicine, Aachen, Germany, 4Department of Neurology, RWTH Aachen University, Aachen, Germany
1Institute of Neuroscience and Medicine 4, INM-4, Forschungszentrum Juelich, Juelich, Germany, 2Institute of Neuroscience and Medicine 11, INM-11, Forschungszentrum Juelich, Juelich, Germany, 3JARA - BRAIN - Translational Medicine, Aachen, Germany, 4Department of Neurology, RWTH Aachen University, Aachen, Germany
Synopsis
The steady development of fMRI techniques has enabled the use of submillimetre-resolution in recent fMRI studies. The use of submillimetre-resolution allows the detection of cortical, depth-dependent brain activation. Although there have been numerous attempts to perform submillimetre-resolution fMRI, the level of spatial resolution in several recent works is still around 0.7 mm. Moreover, most methods do not provide whole-brain coverage. Therefore, this work aims to develop a novel half-millimetre resolution fMRI technique capable of providing whole-brain coverage. Here, the method was employed for exemplary finger-tapping fMRI at 7T and the identification of cortical-depth dependent brain activation was demonstrated.
Introduction
FMRI techniques have been steadily developing to a point where activated brain regions can now be revealed with precise spatial localisation. As a result, submillimetre-resolution is now possible in recent fMRI applications enabling the detection of cortical, depth-dependent brain activation. Although there have been numerous attempts to perform submillimetre-resolution fMRI, the level of spatial resolution in several recent works is still around 0.7 mm.1-6 Moreover, most methods are not dedicated to whole-brain fMRI applications. Therefore, this work aims to provide a novel fMRI technique capable of providing a half-millimetre resolution with whole-brain coverage. The proposed method was developed based on two key elements: a TR-external navigator echo scheme7-9 and EPI with keyhole (EPIK).10-18 Here, finger-tapping fMRI was performed at 7T to carefully examine cortical-depth dependent brain activation in the motor cortex.Methods
FMRI applications require a particular TE to derive strong blood-oxygenation-level-dependent (BOLD) contrast at each field strength. However, this TE constraint often restricts the possibility of increasing the imaging matrix size in EPI, as its echo train length is relatively large. Moreover, in EPI, the navigator echoes, which are often adopted as an internal part of the sequence for the elimination of the N/2 ghost artefacts,19 can also contribute significantly to increasing the TE when the matrix size is relatively large.8,9 A TR-external navigator echo scheme can effectively remove the contribution of navigator echoes on the TE increase by allocating them in a separated low-flip angle excitation (αPC) loop (see Fig. 1a). This scheme was employed here to exploit the advantage it gives in terms of increasing the matrix size for the given TE.Another key element employed in this work is EPIK, which accelerates the original single-shot EPI, as depicted in Fig. 1b, with a similar acquisition strategy to three-shot EPI. However, the segmented, interleaved sampling is only applied to peripheral k-space and full Nyquist sampling is applied for the central k-space, referred to here as “keyhole”. This ensures an optimum SNR and CNR for each temporal frame. Here, the missing k-space lines in the peripheral k-space were reconstructed with sliding-window reconstruction and 48 phase encoding lines were configured as the keyhole region here. In this way, EPIK provides a higher temporal resolution and less geometric distortions while maintaining comparable performance in detecting BOLD signals.10-18
The EPIK readout was combined with the TR-external scheme (see Fig. 1a) and the method was employed in block-based finger-tapping fMRI. A healthy volunteer, screened with a standard safety form with an informed consent, was recruited for the measurement on a Siemens Magnetom Terra 7T scanner with following imaging parameters: TR/TE = 3500/22 ms, FOV = 210 × 210 mm2, matrix = 408 × 408 × 105 slices (0.51 × 0.51 × 1.0 mm3), partial Fourier = 5/8, 3-fold in-plane/3-fold inter-plane (multi-band) acceleration and αPC/αMain = 9°/90°.
Results
Figure 2 shows three representative slices obtained from the proposed method. The images are well reconstructed without any severe degradations and a detailed spatial representation of microstructures (e.g. cortical ribbon) can be observed. Figure 3 shows identified activated voxels at three representative slice locations, obtained with a statistical threshold of a false-discovery-rate (FDR) corrected p-value < 0.0001. The activated voxels are overlaid directly on the reconstructed slices, showing that the identified functional voxels are well localised along the cortical ribbon. Here, particularly for a selected ROI (marked with a rectangle in Fig. 3), a further analysis was carried out to inspect cortical-depth dependent brain activation. As shown in Fig. 4a, line profiles of the t-value were examined with respect to the cortical depth. Here, in total, 20 profiles were examined for the selected grey matter region and their mean ± std plot was computed (see Fig. 4b). The mean plot shows that the t-value was biggest at the pial surface, which was mainly due to the effect of the large-vessel BOLD signals in the gradient-echo EPI sequences.20 However, it was also observed that the t-value, which decreases from the pial surface, starts to increase at a deeper layer location (see ‘P1’) and after reaching its peak point (see ‘P2’), the signal decreases until the WM region. This observation is in line with the results presented in previous cortical-depth dependent fMRI studies.4 Furthermore, for the suppression of the large-vessel BOLD signals, a phase-based correction method20,21 was applied to the acquired data. Figure 4d depicts the corresponding mean ± std plot, showing that the effect of large-vessel BOLD signals at the pial surface was reduced and the behaviour of signal increase at the deeper layer location became more distinct.Discussion and conclusions
This work demonstrates the identification of cortical-depth dependent functional activation using the proposed, half-millimetre, whole-brain imaging technique at 7T. The achieved spatial resolution and brain coverage is substantially larger than the levels presented in other recent high-resolution fMRI studies (see Fig. 5). Figure 5 reveals that the high spatial resolution in the present work was achieved with a relatively large matrix size and a sufficiently large FOV, which is not the case in the previous methods. In particular, the large brain coverage achieved by the proposed method suggests its applicability for more general functional studies such as resting-state fMRI.Acknowledgements
No acknowledgement found.References
- Heidemann RM, Ivanov D, Trampel R, Fasano F, Pfeuffer J, Turner R. Zoomed GRAPPA (ZOOPPA) for Functional MRI. In Proceedings of the 18th Annual Meeting of ISMRM, Stockholm, Sweden, 2010. Abstract 2889.
- Kemper VG, De Martino F, Vu AT, Poser BA, Feinberg DA, Goebel R, Yacoub E. Sub-millimeter T2 weighted fMRI at 7 T: comparison of 3D-GRASE and 2D SE-EPI. Front Neurosci. 2015 May 5;9:163.
- Kok P, Bains LJ, van Mourik T, Norris DG, de Lange FP. Selective Activation of the Deep Layers of the Human Primary Visual Cortex by Top-Down Feedback. Curr Biol. 2016 Feb 8;26(3):371-6.
- Huber L, Ivanov D, Handwerker DA, Marrett S, Guidi M, Uludağ K, Bandettini PA, Poser BA. Neuroimage. Techniques for blood volume fMRI with VASO: From low-resolution mapping towards sub-millimeter layer-dependent applications. 2018 Jan 1;164:131-143.
- Kashyap S, Ivanov D, Havlicek M, Poser BA, Uludağ K. Impact of acquisition and analysis strategies on cortical depth-dependent fMRI. Neuroimage. 2018 Mar;168:332-344.
- Kay K, Jamison KW, Vizioli L, Zhang R, Margalit E, Ugurbil K. A critical assessment of data quality and venous effects in sub-millimeter fMRI. Neuroimage. 2019 Apr 1;189:847-869.
- Wielopolski PA. Echo-planar imaging pulse sequences in Echo-planar imaging theory, technique and application, ed. Schmitt F, Stehling MK, Turner R. 1998. p. 96.
- Yun S, Shah NJ. On the analysis of EPI phase correction with small tip angle excitation to reduce minimum required TE: application to whole-brain submillimetre resolution fMRI at 3T. In Proceedings of the 26th Annual Meeting of ISMRM, Paris, France, 2018. Abstract 4245.
- Yun S, Shah NJ. Full-FOV, Whole-brain, Half-millimetre Resolution fMRI at 7T using Accelerated multi-band EPIK with TR-external Phase Correction. In Proceedings of the 27th Annual Meeting of ISMRM, Montreal, Canada, 2019. Abstract 1167.
- Shah NJ, Zilles K. Verfahren zur Untersuchung eines Objektes mittels Erfassung des Ortsfrequenzraumes. 2003. German Patent Application No. 199 62 845 C2.
- Shah NJ, Zilles K. Imaging process in the spatial frequency space and useful for examining the properties of object. 2004. USA Patent Application No. 6781372 B2.
- Zaitsev M, Zilles K, Shah NJ. Shared k-space echo planar imaging with keyhole. Magn Reson Med. 2001;45(1):109-117.
- Zaitsev M, D'Arcy J, Collins DJ, Leach MO, Zilles K, Shah NJ. Dual-contrast echo planar imaging with keyhole: application to dynamic contrast-enhanced perfusion studies. Phys Med Biol. 2005 Oct 7;50(19):4491-505.
- Yun S, Reske M, Vahedipour K, et al. Parallel imaging acceleration of EPIK for reduced image distortions in fMRI. NeuroImage. 2013;73:135-143.
- Yun S, Shah NJ. Whole-brain high in-plane resolution fMRI using accelerated EPIK for enhanced characterisation of functional areas at 3T. PLoS One. 2017;12(9):e0184759.
- Caldeira LL, Yun S, da Silva NA, Filss C, Shah NJ. Dynamic susceptibility contrast parametric imaging using accelerated dual-contrast echo planar imaging with keyhole. J Magn Reson Imaging. 2019 Aug;50(2):628-640.
- Shah NJ, da Silva NA, Yun S. Perfusion weighted imaging using combined gradient/spin echo EPIK: Brain tumour applications in hybrid MR-PET. Hum Brain Mapp. 2019 Feb
- Yun S, Weidner R, Weiss PH, Shah NJ. Evaluating the Utility of EPIK in a Finger Tapping fMRI Experiment using BOLD Detection and Effective Connectivity. Scientific Reports. 2019;9:article 10978.
- Heid O. Robust EPI phase correction. In Proceedings of the 5th Annual Meeting of ISMRM, Vancouver, British Columbia, Canada, 1997. P. 2014.
- Menon RS. Postacquisition suppression of large-vessel BOLD signals in high-resolution fMRI. Magn Reson Med. 2002 Jan;47(1):1-9.
- Curtis AT, Hutchison RM, Menon RS. Phase based venous suppression in resting-state BOLD GE-fMRI. Neuroimage. 2014 Oct 15;100:51-9.
Figures
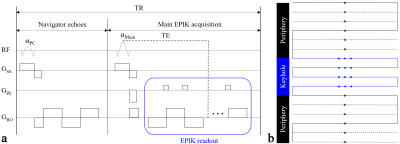
Figure 1. Schematic
representation of (a) a TR-external
navigator echo scheme and (b) a
k-space trajectory for EPIK. Each TR of the TR-external scheme consists of a
low-flip angle excitation (αPC) loop for navigator echoes and a
main excitation (αMain) loop for EPIK readout
(see the blue rectangle in a). The
EPIK acquisition consists of Nyquist sampling (Δky
= 1/FOV)
for central k-space (keyhole) and sparse sampling (Δky'
= 3/FOV)
for peripheral k-space - in which the solid,
dashed and fine-dashed lines indicate positions to be sampled at respective
temporal frame like
three-shot EPI.
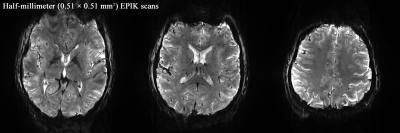
Figure 2. Three representative
reconstructed slices (0.51 × 0.51 mm2) from the proposed method. A
detailed spatial representation of microstructures (e.g. cortical ribbon) can
be observed.
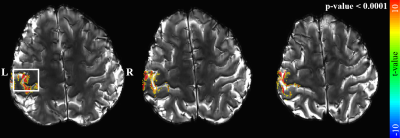
Figure 3. First-level analysis
results for right-index finger-tapping fMRI, obtained with a statistical
threshold of a p-value < 0.0001 (FDR-corrected). The activated voxels are
overlaid on the reconstructed EPIK scans. The results are presented for three
representative axial slice locations. It is observed that the activated voxels
are well localised along the cortical ribbon. For the selected ROI (marked with
a rectangle), a further analysis was carried out to inspect cortical-depth
dependent brain activation.
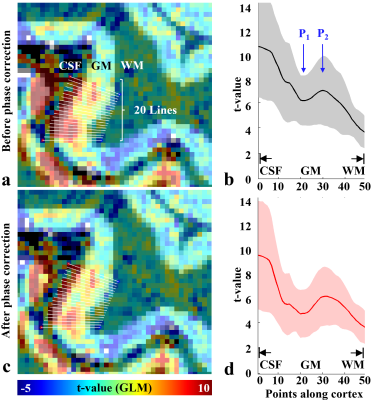
Figure 4. (a) The brain region marked by the white rectangle in Fig. 3, (b) the mean ± std plot of the t-values
at the 20 lines shown in (a), in
which the horizontal axis presents 50 sampling points for the GM (i.e. from the
boundary of CSF to that of WM). The corresponding results after the phase correction
are depicted in (c) and (d), respectively. The functional signal
starts to increase at a deeper layer location (‘P1’) and after
reaching a peak (‘P2’), it decreases until the WM region. This
signal behaviour is more distinct after the phase correction.
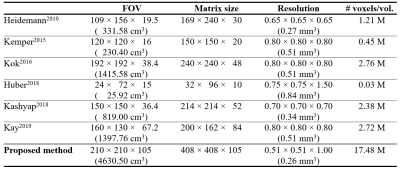
Figure 5. Imaging parameters
employed in previous cortical-depth dependent fMRI studies (1st - 6th rows) and
in this work (last row). This table reveals that the high spatial resolution
(0.51 × 0.51 mm2) of the proposed method was achieved with a
relatively large matrix size (408 × 408) and a sufficiently large FOV (210 ×
210 mm2), which is not the case in the previous methods. The
proposed method also shows significant improvements in the brain volume
(4630.50 cm3) covered by the imaging FOV and the number of voxels
per temporal volume (17.48 M); the voxel volume (0.26 mm3) was also
smallest.