3856
Resolving Spatially Distinct Patterns of Nociception with Functional MRI at 7.0 T En Route to Pain-Specific Neurosignatures1Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, 2Experimental and Clinical Research Center, a joint cooperation between the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany
Synopsis
In recent years, the identification of pain-specific biomarkers has become the holy grail of pain research in the fMRI community. Painful stimuli, salient (attention capturing) non-painful stimuli, and nociceptive stimuli in analgesic subjects (who cannot feel pain) elicit similar BOLD patterns that cannot be resolved at 3T. Recognizing this shortcoming we present preliminary fMRI data at 7T (double spatial resolution), comparing painful stimuli with isosalient non-painful stimuli in healthy volunteers and an analgesic subject who is insensitive to pain. The overall goal of this study is to detail the evoked neurosignatures for pain-specificity and identify diagnostic biomarkers of pain experience.
Introduction
Chronic pain is one of the largest medical health problems (with 35% of adults affected) and the most cost-intensive disease (ICD-11) in the developed world, including medical care, loss of wages and productivity1. The clinical diagnosis of pain still relies entirely on self-reports, which puts an enormous psychological burden on the patients, and proof or disproof of this “invisible” condition can affect payments2. In recent years, the identification of pain-specific biomarkers has become the holy grail of pain research in the fMRI community2,3,4. Brain areas of the so-called “pain matrix” that reliably responds to painful stimuli appear to also respond to other salient (attention capturing) events, including non-painful salient stimuli5,6, nociceptive stimuli in analgesic subjects (who cannot feel pain due to a genetic defect)7, as well as social rejection8. The direct comparison of painful and isosalient innocuous stimuli is a promising strategy to disentangle saliency from pain and reveal pain-specific neurosignatures9,10. Yet, significant differences in spatial pattern characteristics could not be resolved using BOLD fMRI at 3T. Recognizing this shortcoming this work presents preliminary fMRI data aiming at detailing pain-specificity in the brain at 7T with twice the spatial resolution versus previous 3T reports. We compare (1) painful stimuli of different modalities with (2) isosalient non-painful stimuli in healthy volunteers, and (3) contrast the resulting functional patterns with those evoked by the same set of stimuli in an analgesic subject who is insensitive to pain. The overall goal of this study is to detail the evoked neurosignatures for pain-specificity and identify diagnostic biomarkers of pain experience.Methods
Functional MRI was performed after due approval by the local ethical committee. Informed written consent was obtained from each volunteer prior to the study in compliance with the local institutional review board guidelines.Experimental design: Four male volunteers (three controls, one analgesic subject) underwent the fMRI session, consisting of 4 successive runs of 12 min each. Three different types of stimuli (8 per type and run; 9-12 TR interstimulus spacing) were applied in a pseudorandomized manner: Electrical stimulation (100 ms) was applied to the right ankle, over the superficial peroneal nerve in two different stimulus strengths: 6mA and 10 mA. Short pulses of transient heat (51°C) were applied to the dorsum of the right foot.
MR Imaging: High-resolution T2*-weighted fMRI (GE-EPI, TR/TE/FA = 2000ms/28.2ms/66°, FOV/matrix = 240x240mm/160x160mm, number of slices = 80, slice thickness = 1.5mm, spatial resolution = 1.5mm isotropic, 360 volumes) was acquired on a MAGNETOM 7T scanner (Siemens Healthcare, Erlangen, Germany) with a single-channel-transmit/32-channel receive head coil (Nova Medical, Wilmington, MA, USA).
Data analysis: fMRI data were corrected for motion and distortion, smoothed, registered to the MNI152 template, and statistically analyzed using FSL FEAT. Corrected cluster significance thresholds of p<0.05 were determined by z > 3.1.
Results
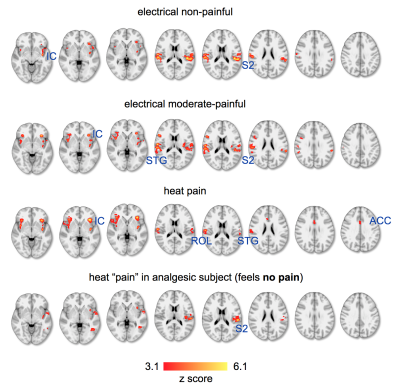
Healthy controls rated heat stimuli (51°C) as painful, electrical stimuli of 10 mA as moderate pain (less painful), and 6mA as salient, but not painful. Saliency (defined to the subjects as “capability of a stimulus to capture the attention”) was reported high for all stimuli. We investigated areas of the pain matrix (which we describe in detail in another submitted abstract) to probe spatial differences between the stimuli (Fig. 1). All stimuli caused bilateral responses in the anterior and posterior insular cortex (IC), superior temporal gyrus (STG), and the secondary somatosensory cortex (S2), including the rolandic operculum (ROL, OP4 of S2). Activity in the insula significantly increased with increasing pain exhibiting highest z-values and BOLD signal magnitudes in response to transient heat. Spatial clusters in S2 evoked by heat were more spatially restricted then those for electrostimulation, which spreads along large areas of S2. Activity in the dorsal anterior cingulate cortex (dACC) has been found only for heat pain.Application of the same transient heat stimuli in the analgesic (“painless”) subject did not show activity in the ACC, and only small and scattered clusters in the contralateral IC (Fig. 1). Also S2 exhibited only contralateral activity, although strong and spatially defined.
Discussion
The results illustrate how areas of the pain matrix are increasingly engaged by increasing pain. The defined patterns in S2 in response to heat stimuli can be explained by specific recruitment of fast Aδ- and slow C- fibers11, whereas electrostimulation engages various fiber types leading to rather unspecific activity. Although all stimuli have been reported salient, we found the ACC only activated in response to heat stimuli. This is in contrast to previous reports on patterns evoked by non-painful salient stimuli that have been shown to include the ACC5,9,10. Since we present here a very small sample size of preliminary data we cannot yet conclude on effects in larger populations. The analgesic (“painless”) subject showed a somatosensory pattern in response to heat stimuli that misses the bilateral distribution that is typical for saliency and pain. This is interesting, since the subject reported the stimulus as clearly salient. We expect to have more clarity by substantially increasing the sample size that permits application of multi-voxel pattern analysis to improve pattern recognition and exploit the benefits of 7T en route to the identification of pain-specific neurosignatures.Acknowledgements
No acknowledgement found.References
1. Steglitz, J. et al. (2012) The future of pain research, education, and treatment: a summary of the IOM report ‘Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Educa- tion, and Research’. Transl. Behav. Med. 2, 6–8
2. Davis, K. D., Flor, H., Greely, H. T., Iannetti, G. D., Mackey, S., Ploner, M., et al. (2017). nrneurol.2017.122. Nature Publishing Group, 13(10), 624–638.
3. Wager, T. D., Atlas, L. Y., Lindquist, M. A., Roy, M., Woo, C.-W., & Kross, E. (2013). An fMRI-Based Neurologic Signature of Physical Pain. New England Journal of Medicine, 368(15), 1388–1397.
4. Woo, C.-W., Schmidt, L., Krishnan, A., Jepma, M., Roy, M., Lindquist, M. A., et al. (2017). Quantifying cerebral contributions to pain beyond nociception. Nature Communications, 8, 14211.
5. Mouraux, A., Diukova, A., Lee, M. C., Wise, R. G., & Iannetti, G. D. (2011). A multisensory investigation of the functional significance of the “pain matrix.” NeuroImage, 54(3), 2237–2249.
6. Mouraux, A., & Iannetti, G. D. (2018). The search for pain biomarkers in the human brain. Brain : a Journal of Neurology, 141(12), 3290–3307.
7. Salomons, T. V., Iannetti, G. D., Liang, M., & Wood, J. N. (2016). The “Pain Matrix” in Pain-Free Individuals. JAMA Neurology, 73(6), 755–756.
8. Lieberman, M. D., & Eisenberger, N. I. (2015). The dorsal anterior cingulate cortex is selective for pain: Results from large-scale reverse inference. Proceedings of the National Academy of Sciences, 112(49), 15250–15255.
9. Liang, M., Su, Q., Mouraux, A., & Iannetti, G. D. (2019). Spatial Patterns of Brain Activity Preferentially Reflecting Transient Pain and Stimulus Intensity. Cerebral Cortex, 29(5), 2211–2227.
10. Su, Q., Qin, W., Yang, Q. Q., Yu, C. S., Qian, T. Y., Mouraux, A., et al. (2019). Brain regions preferentially responding to transient and iso-intense painful or tactile stimuli. NeuroImage, 192, 52–65.
11. Craig, A. D. (2013). Cooling, pain, and other feelings from the body in relation to the autonomic nervous system. Autonomic Nervous System (1st ed., Vol. 117, pp. 103–109). Elsevier B.V.
Figures