3850
Mouse BOLD fMRI of innocuous and putative noxious forepaw stimulation without cardiovascular modulation1Cener for Neuroscience Imaging Research (CNIR), Institute for Basic Science (IBS), Suwon-si, Gyeonggi-do, Korea, Republic of, 2Department of Biomedical Engineering, Sungkyunkwan University, Suwon-si, Gyeonggi-do, Korea, Republic of, 3Department of Health Sciences and Technology, Samsung Advanced Institute for Health Sciences and Technology (SAIHST), Sungkyunkwan University, Suwon-si, Gyeonggi-do, Korea, Republic of
Synopsis
The wide-spread and/or bilateral activity patterns in cortical and thalamic areas were common observation in response to innocuous and noxious electrical stimulation in mouse BOLD fMRI studies. Those findings may be related to the global BOLD effect confounded by stimulus-induced changes in cardiac functions such as heart rate and blood pressure, rather than originated from underlying neural activity. Under the ketamine-xylazine anesthesia, sympathetic activity is known to be suppressed. Here, we measured the response specificity of the BOLD signal responding from innocuous to putative noxious stimuli in mice under ketamine-xylazine anesthesia.
Introduction
The common observations in mouse fMRI of forepaw stimulation under various anesthetics (isoflurane, medetomidine, profopol, urethane, and etomidate) were widespread and/or non-specific activities in cortical and thalamic areas1,2, regardless of unilateral somatosensory or pain stimulation3. Those findings are related to the stimulus-induced changes in cardiac functions such as heart rate and blood pressure induced by the sympathetic activity of the body's fight-or-flight response4. Thus, true sensory- or pain-evoked responses are buried under large bilateral nonspecific cardiovascular responses. Thus, it is critical to suppress cardiovascular responses induced by forepaw stimulation. Since the combination of ketamine and xylazine is known to suppress sympathetic activity5,6, we adopted ketamine-xylazine anesthesia for our mouse fMRI studies7,8. Recently, we observed the robust BOLD response of forepaw stimulation in the contralateral somatosensory networks, similar to neural tracing results8. Here, we measured the stimulus current intensity-dependent BOLD fMRI in mice under ketamine-xylazine anesthesia to test whether the bilateral activity commonly observed during nociceptive stimulation is induced by pain processing or by cardiovascular response.Materials & Methods
Nine C57BL/6 mice (25-29g) were used for fMRI studies on 15.2T/11cm Bruker BioSpec with surface Tx/Rx coil (15mm-id). Mice were anesthetized with a mixture of ketamine and xylazine7,8 during the fMRI experiments.BOLD-fMRI data were acquired using multi-shot GE-EPI sequence with TR for each shot=750ms, TE=11ms, Number of segment=2, flip angle=50°, spatial resolution=125×125x500μm3 and 9 coronal slices. For fMRI studies, electrical pulses width of 0.5 ms at a frequency of 4 Hz were applied to the left forepaw. Stimulus current intensity of 0.1, 0.3, 0.5, and 1.0 mA were interleaved. Each stimulus trial consisted of 30-volume pre-stimulus, 10-volume stimulus, 35-volume inter-stimulus, 10-volume stimulus and 35-volume post-stimulus period. For each stimulus condition, 10-15 fMRI trials were obtained for signal averaging.
During fMRI studies, electrocardiogram and motion-sensitive respiration signals were continuously measured using a physiological monitoring system and recorded using a data acquisition system. From those recordings, signal time courses of heart rate (beats per min) and respiratory rate (bpm; breath per min) were extracted with fast Fourier transformation. Dynamic pulse and respiration data were averaged over repeated fMRI trials in the same fMRI session.
To estimate the regional specificity of sensory-evoked BOLD changes upon stimulus intensity, fMRI data were spatially normalized onto the mouse brain atlas space. Then, time series data were then extracted based on the anatomical ROIs (Fig. 2A) and the percent signal changes over the stimulus duration were averaged to quantify the activation.
Results
Animal-wise averaged physiological data upon stimulus intensity (Fig. 1) showed that the heart rate and the respiratory rate reached 230-270 bpm and no noticeable changes were observed at all of the current intensity ranges. Note that no blood pressure changes were found under ketamine and xylazine anesthesia even by high current forepaw stimulation (data not shown). These show that ketamine suppresses cardiovascular changes. In contrast, the respiratory rate increased 2.5 % relative to the baseline exclusively during forepaw stimulation at 1.0 mA intensity (Fig. 1iv), indicating that current intensity of 1.0 mA is noxious to mice under ketamine and xylazine anesthesia.Somatosensory stimulation induces somatosensory neural activity, while noxious stimulation generally induces both nociceptive neural activity and cardiovascular response such as arterial blood pressure. Using ketamine, artifactual fMRI responses by cardiovascular changes will be removed, leaving only neural activity-induced fMRI responses. In whole brain ROI analysis (Fig. 2A), BOLD responses showed the gradual increase with increasing stimulus intensity in the cortical and thalamic areas contralateral to the stimulated forepaw, while the activation in ipsilateral hemisphere can be detected at current intensity of 1.0 mA (Fig. 2B). The response in contralateral S1FL was significantly higher than that in ipsilateral area (Fig. 2Ci-ii). In the anterior cingulate cortex (ACC) involved in pain processing, the responses were only observed at a stimulus intensity of 1.0 mA (Fig. 2Ciii-iv).
Discussion & Conclusion
In this study, we measured the changes of functional responses upon the stimulus current intensity using high-resolution BOLD fMRI at ultrahigh-field. The major findings were:1) In ketamine-xylazine anesthesia, the fluctuation in respiratory rate is sensitive indicator to noxious stimulation. The usefulness of respiratory rate for pain assessment is supported by clinical findings, which show the strong correlation between response in respiratory rate and self-reported pain intensity9. Compared with awake condition, ketamine and xylazine anesthesia does not depress respiratory, but change cardiovascular functions in mice5. Especially, it reduces heart rate and suppresses sympathetic activity6. Thus, the combination of ketamine and xylazine is ideal for studying fMRI responses to ‘commonly blood pressure-induced’ painful stimuli.
2) As the intensity of the stimulus increases, the response occurs from the sensory-related to pain-related areas, but still shows the laterality of the response (Fig. 3). BOLD responses under ketamine-xylazine anesthesia did not contain the cardiovascular response by sympathetic stimulated blood pressure increase, unlike conventional anesthetics showing a widespread response. Dominant activation in the contralateral hemisphere is likely to reflect true nociceptive processing.
We suggest that the mouse fMRI under ketamine-xylazine will provide insights into understanding functional mechanism from somatosensory to pain processing.
Acknowledgements
This work was supported by IBS-R015-D1.References
1. Petrinovic MM, Hankov G, Schroeter A, et al. A novel anesthesia regime enables neurofunctional studies and imaging genetics across mouse strains. Sci Rep. 2016;6:24523
2. Schroeter A, Schlegel F, Seuwen A, et al. Specificity of stimulus-evoked fMRI responses in the mouse: the influence of systemic physiological changes associated with innocuous stimulation under four different anesthetics. Neuroimage. 2014;94:372-384.
3. Bosshard SC, Baltes C, Wyss MT, et al. Assessment of brain responses to innocuous and noxious electrical forepaw stimulation in mice using BOLD fMRI. Pain. 2010;151(3):655-63.
4. Reimann HM, Todiras M, Hodge R, et al. Somatosensory BOLD fMRI reveals close link between salient blood pressure changes and the murine neuromatrix. Neuroimage. 2018;172:562-574.
5. Arras M, Autenried P, Rettich A, et al. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp Med. 2001;51:443-56.
6. Svorc P Jr, Bačová I, Svorc P, et al. Autonomic nervous system under ketamine/ xylazine and pentobarbital anaesthesia in a Wistar rat model: a chronobiological view. Prague Med Rep. 2013;114(2):72-80.
7. Shim HJ, Jung WB, Schlegel F, et al. Mouse fMRI under ketamine and xylazine anesthesia: Robust contralateral somatosensory cortex activation in response to forepaw stimulation. Neuroimage. 2018;177:30-44.
8. Jung WB, Shim HJ, Kim SG. Mouse BOLD fMRI at ultrahigh field detects somatosensory networks including thalamic nuclei. Neuroimage. 2019;195:203-214.
9. Arbour C, Choinière M,
Topolovec-Vranic J, et al. Can fluctuations in vital signs be used for pain
assessment in critically ill patients with a traumatic brain injury?. Pain Res
Treat. 2014;2014:175794.
Figures
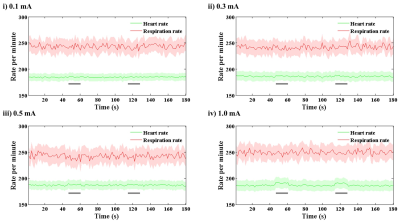
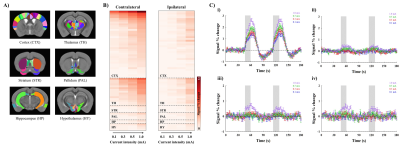
Figure 2. Whole brain ROI analysis to quantify the signal changes during forepaw stimulation.
A) Atlas-based ROI definitions.
B) Regional specificity of BOLD signal changes upon stimulus current intensity.
C) fMRI time courses from the forelimb primary somatosensory S1FL i) contralateral and ii) ipsilateral to stimulated forepaw and iii-iv) anterior cingulate cortex ACC same as S1FL, respectively (mean±SEM). Area in shade indicates the 15 s duration of stimulation.
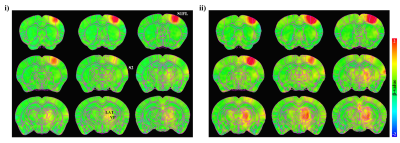
Figure 3. group-averaged fMRI maps without statistical thresholds evoked by stimulus current intensity of i) 0.3 mA and ii) 1.0 mA.
At the lower stimulus intensity, the evoked BOLD response was observed unilaterally in primary somatosensory forelimb (S1FL), secondary somatosensory area (S2) and ventral/lateral group of the dorsal thalamus (VENT, LAT).
The bilateral responses occur at the higher stimulation, but still shows the laterality of the response.