3847
Modulation by cardiac and respiratory cycles of single-trial BOLD responses to visual stimulation using ultrafast fMRI at 7T1ISR-Lisboa/LARSyS and Department of Bioengineering, Instituto Superior Técnico, Universidade de Lisboa, Lisboa, Portugal, Lisbon, Portugal, 2Laboratory for Functional and Metabolic Imaging, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, Lausanne, Switzerland, 3Systems Division, Swiss Center for Electronics and Microtechnology (CSEM), Neuchâtel, Switzerland, Lausanne, Switzerland, 4Spinoza Centre for Neuroimaging, Amsterdam, Netherlands, Amsterdam, Netherlands
Synopsis
The haemodynamic response to neuronal activity measured by BOLD-fMRI exhibits substantial trial-by-trial variability, but the underlying sources remain largely unknown. Here, we investigated whether single-trial BOLD responses were modulated by cardiac and respiratory cycles, by recording the BOLD responses to single trials of brief visual stimulation using an ultrafast acquisition (TR=0.4s) at ultrahighfield (7T). We found that, when binning trials according to the respiratory phase, the BOLD responses were more similar to each other when occurring up to 1sec following expiration. Our results suggest that trial-by-trial variability of BOLD responses may partly be explained by the respiratory cycle.
Introduction
The haemodynamic response to neuronal activity measured by the blood oxygenation-level-dependent (BOLD) signal varies significantly across subjects, sessions, brain regions and within brain regions1-3. Moreover, a few studies analyzed single-trial BOLD responses and reported substantial trial-by-trial variability4-6. This is generally considered to be noise that affects fMRI statistical analysis, and trial averaging is usually performed in order to achieve sufficient SNR. However, there is potentially great interest in the study of single-trials and trial-by-trial variations of brain responses. Although the trial-by-trial variability of BOLD responses is thought to reflect fluctuations in the underlying physiology, as well as with neuronal activity and behavior7, its relationship with the cardiac and respiratory cycles has not been previously reported. Here, we aim to investigate this relationship by using an ultrafast fMRI acquisition (TR=0.4s) at ultrahigh field (7T) to record the individual BOLD responses to single trials of brief visual stimulation simultaneously with cardiac and respiratory recordings.Methods
Data Acquisition5 healthy subjects were studied on a 7T MRI system (Siemens) with a 32-channel head coil. BOLD-fMRI data was collected using whole-brain gradient-echo 3D-EPI-CAIPI8 (TR=0.4s, 2mm isotropic resolution), with simultaneous cardiac and respiratory recordings by a pulse oximeter and a respiratory bellow, while a flickering checkerboard was presented in a slow event-related paradigm (12 trials, 0.5s on/29.5s off) – ER-fMRI dataset. A Localizer-fMRI dataset was also collected using 2D-multislice GE-EPI by presenting the checkerboard in a block design (10 blocks, 10s/20s off).
Data processing
fMRI datasets were analyzed using FSL (fsl.fmrib.ox.ac.uk/fsl). Pre-processing included motion correction, high-pass temporal filtering (cutoff=50s), spatial smoothing (FWHM=3mm), and regression of 2nd order cardiac and respiratory fluctuations using RETROICOR9. A GLM analysis of the Localizer dataset was performed to obtained a map of voxels significantly responding to the visual stimulus. An ROI was defined in the primary visual cortex of each subject, by thresholding the statistical map (Z>6) and registering it to the ER-fMRI space. The pre-processed ER-fMRI BOLD signal was averaged across the ROI, and this timecourse was then segmented into the 12 trials, for each subject.
Trial-by-trial analysis
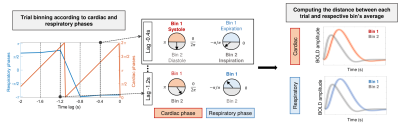
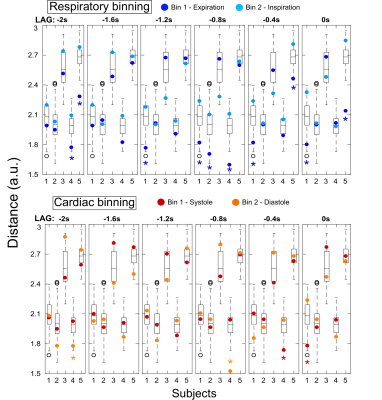
We hypothesized that, if cardiac/respiratory cycles influence the BOLD response on a trial-by-trial basis, then the single-trial BOLD responses occurring at similar phases of the cardiac/respiratory cycles should be more similar with each other than those occurring at different phases. In order to test this hypothesis, we binned the trials according to cardiac/respiratory phases, and tested whether the distance between trials within each bin was smaller compared to random bins. The phase of the cardiac/respiratory cycles was computed9, for each trial of each subject, at the stimulus onset and at time lags up to 2sec preceding it (lags=0,-0.4,-0.8,-1.2,-1.6 and -2.0sec). The 12 trials of each subject were grouped into two bins (Bin1/Bin2) based on their respective cardiac/respiratory phases at each time lag, for each physiological signal (cardiac/respiratory) (Fig.1). For each subject, physiological signal, lag and bin, the Euclidean distance was computed between each trial’s BOLD signal and the averaged BOLD signal across all trials in the bin; the mean distance across all trials in each bin was then obtained. We generated surrogate data for each subject by randomly grouping the 12 trials into a bin of 6 trials, and repeated this 900 times. The same procedure was applied to obtain the mean distance for each random bin, yielding the surrogate distribution for each subject. Z scores were computed for the mean distances of each bin against the respective surrogate distribution.
Results
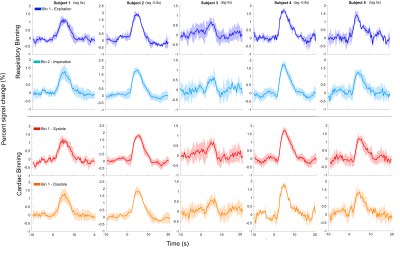
The mean distances for each bin are presented with the respective surrogate distribution in Fig.2. When binning trials according to respiratory phases, during expiration, 4 out of 5 subjects showed significantly smaller bin distances for at least one time lag up to 1sec before the stimulus onset. At the same time lags a trend could also be observed for greater distances during inspiration. When binning according to the cardiac cycle, no consistent pattern was found either related to the systole or diastole. The BOLD signal time courses of the trials binned according to cardiac/respiratory phases at selected time lags, for each subject, are shown in Fig.3.Discussion and conclusion
Our results suggest that trial-by-trial variability of BOLD responses may be modulated by the respiratory cycle, specifically that the hemodynamic response is less variable when subjects exhale up to 1sec before the stimulus onset. This could be explained by differences in the haemodynamic response as a function of the basal state of the vasculature, which in turn is influenced by the respiratory cycle and associated variations in arterial CO2, a powerful vasodilator. This work motivates further research focusing on the cardiac and respiratory cycles as a potential source of trial-by-trial BOLD variability.Acknowledgements
We acknowledge funding by the Portuguese Science Foundation (FCT) through Project UID/EEA/50009/2013, the Centre d'Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL and the Leenaards and Jeantet Foundations.References
1. G. K. Aguirre, E. Zarahn, and M. D’esposito. The variability of human, BOLD hemodynamic responses. Neuroimage. 1998;8:360–369.
2. D. A. Handwerker, J. M. Ollinger, and M. D’Esposito. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage. 2004;21:1639–1651.
3. Z. S. Saad, K. M. Ropella, R. W. Cox, et al. Analysis and use of fMRI response delays. Human brain mapping. 2001;13:74–93.
4. Duann, J. R., Jung, T. P., Kuo, W. J., et al. Single-trial variability in event-related BOLD signals. Neuroimage. 2002;15(4):823-835.
5. Fox, M. D., Snyder, A. Z., Zacks, J. M., & Raichle, M. E. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nature neuroscience. 2006;9(1):23.
6. Mayhew, S. D., Mullinger, K. J., Ostwald, D et al. Global signal modulation of single-trial fMRI response variability: Effect on positive vs negative BOLD response relationship. Neuroimage. 2016;133: 62-74.
7. Fox, M. D., Snyder, A. Z., Vincent, J. L., et al. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171-184.
8. Narsude M., Gallichan G., van der Zwaag W., et al. Three-dimensional echo planar imaging with controlled aliasing: a sequence for high temporal resolution functional MRI. Magnetic Resonance in Medicine. 2015;75(6):2350-2361.
9. Glover, G. H., Li, T. Q., & Ress, D.. Image‐based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magnetic Resonance in Medicine. 2000;44(1):162-167.
Figures