3846
Vascular effects in fMRI during respiratory-gated auricular vagal nerve stimulation
Nikos Priovoulos1, Dimo Ivanov1, Benedikt Poser1, Linda Pagen1, Vitaly Napadow2,3, Roberta Sclocco2, Frans Verhey1, and Heidi Jacobs1,2
1Maastricht University, Maastricht, Netherlands, 2Harvard Medical School, Boston, MA, United States, 3Logan University, Chesterfield, MT, United States
1Maastricht University, Maastricht, Netherlands, 2Harvard Medical School, Boston, MA, United States, 3Logan University, Chesterfield, MT, United States
Synopsis
Vagus nerve stimulation has been suggested to cause wide-spread changes in the hemodynamic response function in rats. Here, we demonstrate in humans that a non-invasive variant of vagus nerve stimulation is linked with neurovascular and global blood flow changes, and associated with increased salivary alpha-amylase, suggesting potential noradrenaline release. Our results provide support for the role of noradrenaline in modulating BOLD fMRI contrast via blood flow.
Introduction
Vagus nerve stimulation (VNS) is a neuromodulation method that applies electrical current over the vagus nerve. VNS and its non-invasive variants have several purported clinical effects, ranging from amelioration of depression to memory enhancement [1, 2], however its mechanism is unclear. In rats, a widespread modulation of the hemodynamic response function during VNS has been shown [3], though no such effects have been examined in humans. VNS is thought to stimulate, amongst others, the locus coeruleus, a brainstem nucleus that increases noradrenaline release [4]. Noradrenaline is taken up by receptors of the sympathetic nerves lining the vasculature, causing vasoconstriction [5, 6]. The sympathetic system has been hypothesized to affect the BOLD signal by modulating blood flow [7], but this has not been shown in humans. In this study, we examined the vascular contributions to the fMRI signal during the application of a non-invasive VNS method (respiratory-gated auricular vagal afferent nerve stimulation; RAVANS [8-10] in humans.Methods
MRI scans (N=17 aged adults; median (IQR)=71.4 (65.3-74.8) years; 8 male) were performed using a 7T Siemens scanner with 32-channel Nova head coil. The experiment consisted of 6min of continuous fMRI during which RAVANS or Sham were continuously administered (randomized order, 2 sessions). Custom MR-compatible electrodes were placed in the cymba concha of the left ear. RAVANS was applied with 200μs long 5mA pulses at 8Hz (TENS-dental stimulator), gated for 1s during exhalation (Fig. 1). Sham consisted of stimulation only in the first and last 30s of the 6min period. T2*-weighted EPI (TR/TE=2000/17ms, SMS/MB=3x2, voxel-size=1.25mm isotropic, 50 slices) was recorded to evaluate BOLD fMRI signal during stimulation. Concurrently, photoplethysmography (PPG; left finger) was measured. Salivary samples, from which alpha-amylase (sAA; a noradrenaline proxy [11]) was derived, were acquired before, after and 12 min after RAVANS. The BOLD fMRI data were motion, distortion and ICA-FIX denoised. The PPG's envelope reflects pulsatile changes by measuring local blood volume change and oxyhemoglobin content: PPG amplitude drops have been linked to global BOLD oscillations [7, 12]. The peaks in the PPG signal were detected after bandpass filtering at [0, 2Hz]. The envelope of the PPG signal was extracted and equidistantly sampled to the PPG sampling rate (50Hz). The PPG envelope was used as a proxy of cerebral blood volume: a correlation analysis was run between PPG and BOLD, masked for vein regions extracted from [13]. A within-participant GLM (RAVANS>Sham; matched for respiration phase) was fitted and a RAVANS group-average effect was calculated. The sAA values before and after the stimulation were compared with Wilcox-tests.Results
PPG showed an amplitude drop after stimulation events (~6-7sec), suggesting a stimulation-locked blood volume decrease in the finger. This can be observed in the Sham condition, where bursts of RAVANS induced an amplitude modulation over a period of ~30sec (Fig. 2A-C). Using the PPG envelope as a regressor showed a cluster of increased correlation during RAVANS between PPG and BOLD fMRI signal in large veins (Fig. 3A-B). Stimulation events induced a decreased response to the venous BOLD (Fig. 3C). A similar effect can be seen in the global BOLD signal (Fig. 4F). Increased sAA following stimulation was also detected, in agreement with a hypothesized increased release of noradrenaline (ZWilcox=87, p-value=0.03; Fig. 4C). The venous BOLD variability negatively correlated to sAA only during continuous RAVANS stimulation (RSpearman=-0.82, p-value=0.02, Fig. 4D). This was also noted for global BOLD variability (RSpearman=-0.93, p-value=0.002, Fig. 4F).Discussion
Discussion VNS can introduce widespread changes in the BOLD signal in rats [3]. Here, we demonstrate in humans that this may relate to neurovascular changes: RAVANS introduced blood-volume variations in humans that were detectable via PPG in the finger. This correlated to the venous BOLD signal and a simultaneous change in global BOLD signal. We suggest that this may relate to noradrenaline release: VNS induced increases in sAA. Interestingly, arousal-mediated blood volume decreases have been suggested to underlie resting-state fluctuations [7, 12]. Noradrenaline release from the locus coeruleus may support this effect: noradrenaline stimulates the cerebral arterial sympathetic neurons, inducing vasoconstriction, amongst other effects [6]. Here, we add evidence for this mechanism by demonstrating that increased sAA (noradrenaline proxy) correlates to venous and global BOLD signal variability. The observed venous and global BOLD signal decrease following stimulation may relate to a reduction in blood-oxygenation, due to upstream arterial constriction [7].Conclusion
To sum up, we suggest that noradrenaline release may affect the BOLD signal by modulating vasoconstriction. We confirm in humans that the underlying mechanisms of RAVANS include its influence on the noradrenergic system, leading to vascular and wide-spread BOLD signal changesAcknowledgements
No acknowledgement found.References
1. Trevizol, A.P., et al., Transcutaneous vagus nerve stimulation (tVNS) protocol for the treatment of major depressive disorder: A case study assessing the auricular branch of the vagus nerve. Epilepsy & Behavior, 2015. 53: p. 166-167. 2. Jacobs, H.I.L., et al., Transcutaneous vagus nerve stimulation boosts associative memory in older individuals. Neurobiology of Aging, 2015. 36(5): p. 1860-1867. 3. Cao, J., et al., Vagal nerve stimulation triggers widespread responses and alters large-scale functional connectivity in the rat brain. PLoS One, 2017. 12(12): p. e0189518. 4. Samuels, E.R. and E. Szabadi, Functional Neuroanatomy of the Noradrenergic Locus Coeruleus: Its Roles in the Regulation of Arousal and Autonomic Function Part I: Principles of Functional Organisation. Current Neuropharmacology, 2008. 6(3): p. 235-253. 5. Bekar, L.K., H.S. Wei, and M. Nedergaard, The locus coeruleus-norepinephrine network optimizes coupling of cerebral blood volume with oxygen demand. Journal of Cerebral Blood Flow & Metabolism, 2012. 32(12): p. 2135-2145. 6. Goadsby, P.J., G.A. Lambert, and J.W. Lance, The mechanism of cerebrovascular vasoconstriction in response to locus coeruleus stimulation. Brain Research, 1985. 326(2): p. 213-217. 7. Ozbay, P.S., et al., Contribution of systemic vascular effects to fMRI activity in white matter. Neuroimage, 2018. 176: p. 541-549. 8. Garcia, R.G., et al., Modulation of brainstem activity and connectivity by respiratory-gated auricular vagal afferent nerve stimulation in migraine patients. Pain, 2017. 9. Napadow, V., et al., Evoked pain analgesia in chronic pelvic pain patients using respiratory-gated auricular vagal afferent nerve stimulation. Pain Med, 2012. 13(6): p. 777-89. 10. Sclocco, R., et al., Neuroimaging brainstem circuitry supporting cardiovagal response to pain: a combined heart rate variability/ultrahigh-field (7 T) functional magnetic resonance imaging study. Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences, 2016. 374(2067). 11. Schwab, K.O., G. Heubel, and H. Bartels, Free epinephrine, norepinephrine and dopamine in saliva and plasma of healthy adults. Eur J Clin Chem Clin Biochem, 1992. 30(9): p. 541-4. 12. Tong, Y., et al., Evaluating the effects of systemic low frequency oscillations measured in the periphery on the independent component analysis results of resting state networks. Neuroimage, 2013. 76: p. 202-15. 13. Huck, J., et al., High resolution atlas of the venous brain vasculature from 7 T quantitative susceptibility maps. Brain Struct Funct, 2019. 224(7): p. 2467-2485.Figures
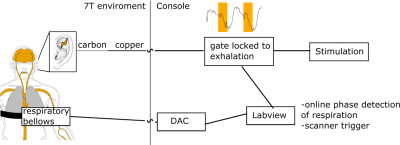
Figure1: RAVANS setup. The stimulation is gated by the
respiration phase to maximize the stimulation effect [8, 10].
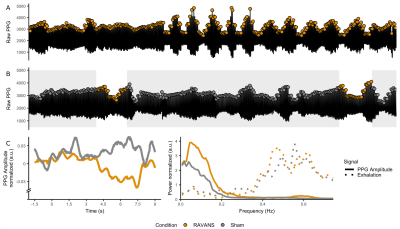
Figure 2: PPG response for RAVANS
(yellow) and Sham (gray). A-B: Raw PPG signal for single participant. The PPG
amplitude in RAVANS, both in the stimulation condition and the stimulation
periods of the Sham condition showed amplitude drops. C, Group PPG peristimulus
plot. A drop in PPG amplitude occurs 7.5 sec after RAVANS events. F, Group-wise
power spectral density of respiration (dashed) and PPG amplitude (solid). The power
of PPG envelope increases for RAVANS at 0.06 Hz.
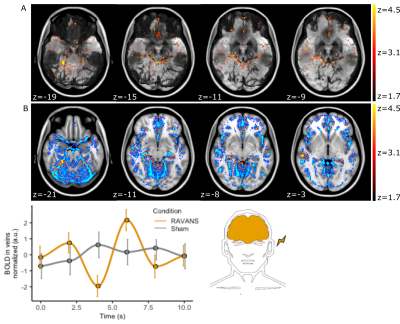
Figure 3: Increased BOLD
correlation to PPG envelope during RAVANS. A, z-map for a single participant
overlaid on a minimum-intensity-projection of the T2*-EPI to enhance
vessel visualization (black). Note the overlap of the cluster to individual
vessel anatomy. B, Group-level cluster overlaid on an atlas of major veins. C, Group-averaged
BOLD vein signal following stimulation (at 0.0 s). A BOLD signal decrease is
observed at 4sec followed by an increase.
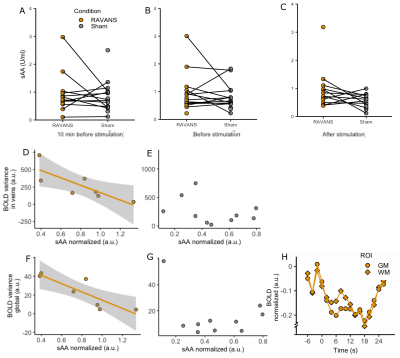
Figure4: A-C. sAA per timepoint and
condition. A significant increase was found after the stimulation specifically
(C). D-G, sAA correlation to venous and global BOLD variability. D,F. RAVANS. A
significant decrease of BOLD variance was found with increased sAA for both
venous (D) and global (F) BOLD. E-G. Sham. No correlation was found. F.
Peristimulus plot of global signal around RAVANS events (white matter and grey
matter).