3845
Anesthetized Mouse fMRI and optical imaging under spontaneous-breathing vs. mechanical ventilation1Center for Neuroscience Imaging Research (CNIR), Institute for Basic Science (IBS), Suwon-si, Korea, Republic of, 2Department of Health Sciences and Technology, Samsung Advanced Institute for Health Sciences and Technology (SAIHST), Sungkyunkwan University (SKKU), Seoul, Korea, Republic of, 3Department of Biomedical Engineering, Sungkyunkwan University (SKKU), Suwon-si, Korea, Republic of
Synopsis
Evoked fMRI findings of anesthetized mice are inconsistent possibly due to uncontrolled vascular physiology. In our studies under ketamine and xylazine anesthesia, localized BOLD response was observed in the spontaneously breathing condition, which is known to cause hypercapnia. Here we performed blood gas analysis and functional studies of spontaneously breathing vs. mechanical ventilating mice. The mechanical ventilation maintained mice at normal physiology and induced larger hemodynamic and BOLD responses to forepaw stimulation. Spontaneous breathing induced severe hypercapnia and acidosis, but surprisingly showed significant evoked functional responses. These results suggest that both methods can be used for functional experiments.
Introduction
BOLD fMRI of anesthetized mouse has been of a great interest, but its findings are inconsistent among literature [1-3]. Typically, no arterial sampling has been performed for mouse fMRI studies due to a small body size, so vascular physiology cannot be controlled precisely. This might be the reason of variabilities of fMRI findings. In our previous mouse fMRI studies under spontaneous breathing, a ketamine and xylazine mixture was successfully used for obtaining localized functional activities responding to forepaw stimulation [4, 5]. It is known that spontaneous breathing under ketamine induces hypercapnia [6]. Especially high carbon dioxide levels elevate the basal cerebral blood flow with vasodilation of cerebral blood vessels, consequently inducing the reduced magnitude and sluggish dynamics of BOLD responses [7]. Thus, we have investigated whether different pCO2 levels can change hemodynamic responses evoked by forepaw stimulation in ketamine/xylazine anesthetized mice.Methods
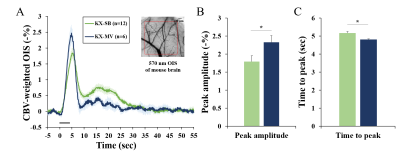
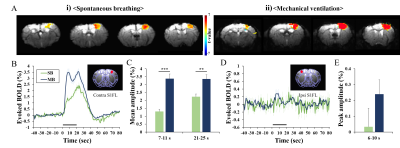
Fifty eight male C57BL/6 mice were used under ketamine and xylazine anesthesia: 8 for physiology measurements, 18 for optical studies, and 22 for fMRI studies. Spontaneous breathing (KX-SB) and mechanical ventilation (KX-MV) were used for evaluating the impact of breathing methods to vascular physiology and functional studies. In all studies, the same length of tubing was used to translate physiological findings with arterial blood gas analysis to fMRI studies (which requires long tubing from the ventilator to the animal inside the magnet). For mechanical ventilation, tracheal intubation was performed (22G catheter) and ventilator settings were determined with extensive preliminary studies to maintain a normal pCO2 level of ~40 mmHg. Femoral artery was catheterized to withdraw blood and measure blood pressure; and blood gases, arterial blood pressure and heart rate were measured (Table 1). To investigate vascular reactivity under the hypercapnic KX-SB condition, vascular reactivity was measured with acetazolamide.Two functional studies were performed with single-forepaw electric stimulation (0.5ms, 0.5mA, 4Hz) for hypercapnic KX-SB vs. normocapnic KX-MV condition. CBV-weighted intrinsic optical imaging (OIS) was performed on MVX-10 system, and BOLD fMRI was measured at 9.4T/30-cm Bruker Biospin with GE-EPI (TR/TE = 16/1000ms, 188x188x500µm3).
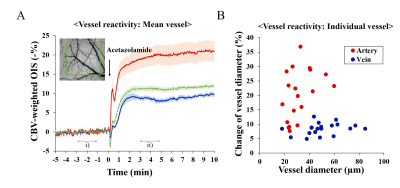
Diameter of pial arterial and venous vessels was calculated responding to the intravenous injection of acetazolamide, and functional signals responding to forepaw stimulation were determined from the primary somatosensory cortical ROI.
Results and Discussion
Baseline physiological values measured during two different breathing conditions are reported in Table 1. During spontaneous breathing, pCO2 level is extremely high, while blood pressure and oxygen saturation level are normal. Under severe hypercapnia, it is expected that vascular reactivity is severely hampered due to dilation of arterial vessels. However, the vascular reactivity is intact in the KX-SB group (Fig. 1), suggesting further dilation by stimulation is feasible. Larger arterial vessels dilate more, while venous vessels respond independent of vessel size. Evoked CBV-weighted OIS response to 4-s forepaw stimulation was detected in all tested mice for both conditions (Fig. 2). Clearly, hypercapnia reduced peak amplitude and slowed down evoked CBV-weighted responses. Similarly, reduced and slower BOLD signal changes responding to 20-s forepaw stimulation were observed in the primary somatosensory cortex under spontaneous breathing than those under mechanical ventilation (Fig. 3). Activation was localized at the contralateral somatosensory cortex for both conditions, but higher statistical values were detected under the mechanical ventilation.The mechanical ventilation is advantageous to have higher functional responses to neural stimulation, but disadvantageous for the requirement of intubation, which risks suffocation and injury. In fact, our intubated mice did not gain normal weight of 1-2 g per week after they recovered from the anesthesia, so were not used for repeated experiments within one week. The most important adverse effect of ketamine/xylazine anesthesia is severe hypercapnia and acidosis due to the impaired gas exchange. However, anesthetized mice were successfully recovered from the anesthesia, and were able to quickly gain weight. Consequently, spontaneous breathed mice were used for another studies in a week later. Reduced magnitude and sluggish functional responses under the hypercapnic spontaneous breathing condition are consistent with human fMRI findings [7].
Conclusion
Spontaneous breathing condition for ketamine/xylazine anesthetized mice induces severe hypercapnia at baseline, but significant vascular reactivity and evoked functional responses. Thus, even though mechanical ventilation provides higher functional responses, spontaneous breathing is still viable for fMRI studies due to its simplicity.Acknowledgements
This work supported by the Institute for Basic Science (IBS-R15-D1)References
1. Reimann, H.M., et al., Somatosensory BOLD fMRI reveals close link between salient blood pressure changes and the murine neuromatrix. NeuroImage, 2018. 172: p. 562-574.
2. Adamczak, J.M., et al., High field BOLD response to forepaw stimulation in the mouse. Neuroimage, 2010. 51(2): p. 704-712.
3. Schroeter, A., et al., Specificity of stimulus-evoked fMRI responses in the mouse: the influence of systemic physiological changes associated with innocuous stimulation under four different anesthetics. Neuroimage, 2014. 94: p. 372-384.
4. Jung, W.B., H.-J. Shim, and S.-G. Kim, Mouse BOLD fMRI at ultrahigh field detects somatosensory networks including thalamic nuclei. NeuroImage, 2019. 195: p. 203-214.
5. Shim, H.-J., et al., Mouse fMRI under ketamine and xylazine anesthesia: Robust contralateral somatosensory cortex activation in response to forepaw stimulation. Neuroimage, 2018. 177: p. 30-44.
6. Arras, M., et al., Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comparative medicine, 2001. 51(5): p. 443-456.
7. Cohen, E.R., K. Ugurbil, and S.-G. Kim, Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. Journal of Cerebral Blood Flow & Metabolism, 2002. 22(9): p. 1042-1053.
Figures