3829
Improving breath-hold cerebrovascular reactivity mapping with multi-echo BOLD fMRI1Basque Center on Cognition Brain and Language, San Sebastián, Spain, 2Physical Therapy and Human Movement Sciences, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States, 3Biomedical Engineering, McCormick School of Engineering, Northwestern University, Evanston, IL, United States
Synopsis
Cerebrovascular reactivity (CVR) is an emerging metric for quantifying vascular health. During a BOLD fMRI acquisition, breath-holding is a simple approach for increasing blood CO2 levels, thereby driving the vasodilatory CVR response. However, breath-holding is associated with increased head motion artifacts, which can confound our estimates of CVR. We demonstrate that multi-echo BOLD fMRI acquisitions, in conjunction with denoising with multi-echo independent component analysis approaches, enable better differentiation of true vasodilatory responses and improve the reliability of CVR estimates across multiple sessions in highly sampled individuals.
Introduction
Cerebrovascular reactivity (CVR) measures the ability of the brain’s vasculature to respond to a vasodilatory stimulus, such as CO2, and is an emerging imaging metric of cerebrovascular health. Breath-Hold (BH) induced CVR mapping is a valid alternative to gas inhalation challenges1 with functional MRI. However, BH movement artifacts are time-locked with the vasodilatory signal of interest, potentially introducing considerable bias on CVR estimates. Multi-Echo (ME) BOLD fMRI enhances the sensitivity to the BOLD effect by optimally combining the echoes, and enables denoising approaches that have demonstrated to remove non-BOLD (e.g. movement) artefacts effectively2,3,4,5.Using a combined ME BOLD and pseudo-continuous arterial spin labelling acquisition, Cohen and Wang have shown that Optimal Combination (OptCom) of ME time series improves the reliability and repeatability of BH induced CVR mapping6 assessed over 2 sessions. However, OptCom is not sufficient to remove motion effects from true fluctuations related to CVR. Here, we (1) generalise these results by computing quantitative CVR maps obtained from a fast (1.5 s) TR, ME BOLD acquisition acquired over 9 sessions with concurrent end-tidal CO2 recordings, and (2) compare how single echo, OptCom and ME-based Independent Component Analysis (ME-ICA) denoising3,4,5 preprocessing pipelines remove motion artefacts and obtain more reliable results.
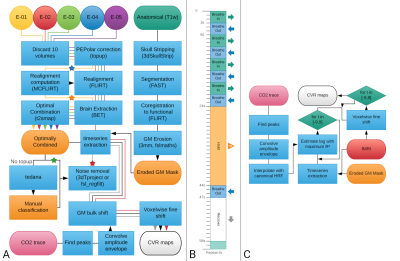
Methods
Three healthy subjects underwent nine imaging sessions in a 3T Siemens PrismaFit scanner, spaced 1 week apart at the same time of day. A BH task7 was administered at the end of each session (Figure 1B) while collecting ME-fMRI data (340 scans, TR=1.5 s, TEs=10.6/28.69/46.78/64.87/82.96 ms, flip angle=70°, Multi-Band=4, GRAPPA=2, 52 slices, Partial-Fourier=6/8, FoV read=211x211 mm, voxel size = 2.4x2.4x3 mm3). CO2 levels were measured using a nasal cannula with an ADInstruments ML206 gas analyser connected to a BIOPAC MP150 system. A T1-w MP2RAGE image was also collected (176 slices, FoV read = 256 mm, voxel size = 1x1x1 mm3) during each session.ME-fMRI preprocessing (see Figure 1A) was done for the second-echo (echo-2), OptCom and two types of denoising were implemented after ME-ICA decomposition with TEDANA8: partial regression that considers the full mixing matrix (pr-meica) and orthogonalization of the rejected “noise” components’ time series with respect to the accepted ones (ort-meica). ME-ICA returned over 250 independent components for all the subjects; these were manually classified and over 200 components were rejected for each dataset.
For each preprocessing pipeline, CVR maps in units of %BOLD/mmHg end-tidal CO2 were calculated as previously described7 (Figure 1) and masked by both statistical significance (p<0.01, two-tailed) and physiological plausibility (absolute temporal shift <= 8.75 s) of the CO2 model fit.
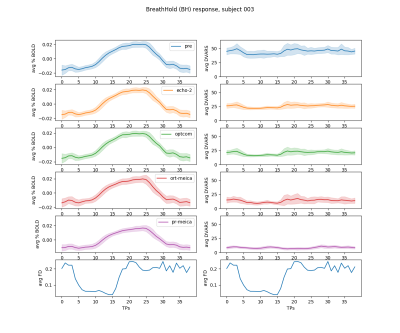
To assess the effectiveness of each type of preprocessing for motion removal, Framewise Displacement (FD) was computed from the realignment parameters, and DVARS was computed before (pre) and after (echo-2) realignment, and after OptCom, pr-meica, and ort-meica. Correlations between FD and each DVARS were computed within each subject and each session.
Reliability of the average grey matter CVR values between subjects (“general”) and of the spatial patterns of CVR within subjects (“spatial”) was assessed using ICC(1,1)10,11. For each subject, session-specific CVR maps were realigned with the first session, and then a mask of grey matter voxels was used to extract the voxels of interest. For both types of ICC, the 9 sessions were considered as a randomly extracted sample of all possible sessions of a subject10.
Results and discussion
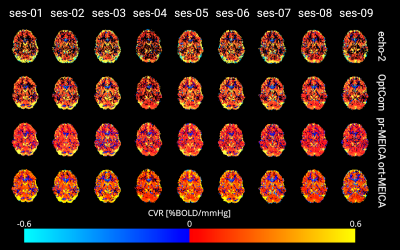
DVARS and FD show a large correlation in the echo-2 and OptCom preprocessing pipelines, suggesting the relevance of widespread intensity changes related to head motion and calling for further denoising that was not applied here. After ME-ICA the effectiveness in reducing motion-related artefacts depends on how nuisance regression is performed on the OptCom data: ort-meica demonstrates some degree of improvement, whereas considering all of the components in the regression model during pr-meica inherently makes DVARS orthogonal to FD (see Figure 2) and reduces the amplitude of DVARS in average and during BH, although the CVR responses are very similar regardless of the type of preprocessing after averaging across the 72 trials of all sessions (see Figure 3).Figure 4 shows the CVR maps across the 9 sessions for all types of preprocessing for a representative subject. Compared to echo-2, CVR maps obtained from OptCom and ME-ICA preprocessing show higher statistical power and improved physiological plausibility of the CVR estimates, while also reducing spurious negative values.
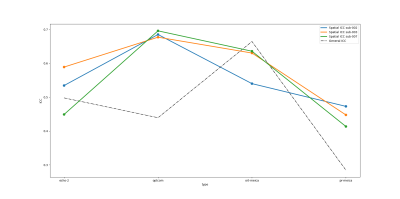
Figure 5 depicts the spatial and general reliability of the CVR estimates using ICC(1,1) values. As predicted, part of the unstructured noise is removed by optimally combining the ME data, increasing spatial reliability. However, the more motion artefacts are removed from the data with ort-meica and pr-meica respectively, the worse the spatial reliability of the CVR maps appeared, indicating a possible contribution of structured noise to the original reliability estimates. Head movement related to breathing tasks likely contributes reliable patterns of confounding artifacts in CVR data, further motivating the incorporation of advanced denoising such through ME-ICA. Interestingly, general ICC is reduced following pr-meica; this may reflect methodological concerns associated with removing a high number of “noise” components that may partially reflect our signal of interest. Alternatively, this may indicate we are better measuring true physiologic variability in CVR occurring over a 9-week study.
Acknowledgements
Research reported in this abstract was supported by the European Union’s Horizon 2020 research and innovation programme (Marie Skłodowska-Curie grant agreement No. 713673), a fellowship from La Caixa Foundation (ID 100010434, fellowship code LCF/BQ/IN17/11620063), the Spanish Ministry of Economy and Competitiveness (Ramon y Cajal Fellowship, RYC-2017-21845), the Spanish State Research Agency (BCBL “Severo Ochoa” excellence accreditation, SEV- 2015-490), the Basque Government (BERC 2018–2021), the Spanish Ministry of Science, Innovation and Universities (MICINN; FJCI-2017-31814), the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number K12HD073945.References
1. Kastrup A, Krüger G, Neumann-Haefelin T, Moseley ME. Assessment of cerebrovascular reactivity with functional magnetic resonance imaging: Comparison of CO2 and breath holding. Magn Reson Imaging. 2001;19(1):13-20. doi:10.1016/S0730-725X(01)00227-2
2. Caballero-Gaudes C, Reynolds RC. Methods for cleaning the BOLD fMRI signal. Neuroimage. 2017;154(December 2016):128-149. doi:10.1016/j.neuroimage.2016.12.0183.
3. Kundu P, Voon V, Balchandani P, Lombardo MV, Poser BA, Bandettini PA. Multi-echo fMRI: a review of applications in fMRI denoising and analysis of BOLD signals. Neuroimage. 2017 Jul 1;154:59-80.
4. Kundu P, Inati SJ, Evans JW, Luh W-M, Bandettini PA. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage. 2012;60:1759–1770. doi:10.1016/j.neuroimage.2011.12.0285.
5. Power JD, Plitt M, Gotts SJ, Kundu P, Voon V, Bandettini PA, Martin A. Ridding fMRI data of motion-related influences: Removal of signals with distinct spatial and physical bases in multiecho data. Proceedings of the National Academy of Sciences, 115(9):E2105-14. doi:10.1073/pnas.1720985115
6. Cohen AD, Wang Y. Improving the Assessment of Breath-Holding Induced Cerebral Vascular Reactivity Using a Multiband Multi-echo ASL/BOLD Sequence. Sci Rep. 2019;9(1):1-12. doi:10.1038/s41598-019-41199-w
7. Bright MG, Murphy K. Reliable quantification of BOLD fMRI cerebrovascular reactivity despite poor breath-hold performance. Neuroimage. 2013;83:559-568. doi:10.1016/j.neuroimage.2013.07.007
8. DuPre E, Salo T, Markello R, Kundu P, Whitaker K, Handwerker D. ME-ICA/tedana: 0.0.6. February 2019. doi:10.5281/ZENODO.2558498
9. Smyser CD, Snyder AZ, Neil JJ. Functional connectivity MRI in infants: Exploration of the functional organization of the developing brain. Neuroimage. 2011;56(3):1437-1452. doi:10.1016/j.neuroimage.2011.02.073
10. Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull. 1979;86(2):420-428. doi:10.1037/0033-2909.86.2.420
11. Cicchetti D V. Interreliability Standards in Psychological Evaluations. Psychol Assess. 1994;(4):284-290.
Figures