3827
Locally reduced cerebral blood flow in patients with Idiopathic Pulmonary Fibrosis1Cardiff University Brain Research Imaging Centre (CUBRIC), Cardiff University, Cardiff, United Kingdom, 2Respiratory Medicine, Cardiff and Vale University Health Board, Cardiff, United Kingdom, 3Institute for Advanced Biomedical Technologies, Department of Neuroscience, Imaging and Clinical Sciences, "G. D'Annunzio University" of Chieti-Pescara, Chieti, Italy
Synopsis
Idiopathic Pulmonary Fibrosis (IPF) is a palliative lung condition. Neuroimaging has the potential to shed light on the neural pathways of cough, a common, troublesome symptom in IPF. However, given the nature of the condition, it is important to first understand the physiological state of brain tissue before investigating functional networks. No significant differences in brain volume were observed in IPF patients but a localised reduction in resting perfusion was seen. There was no difference in functional response when performing a combined motor and visual task. The results support future neuroimaging investigation into the cough pathway in IPF.
Introduction / Purpose
Idiopathic Pulmonary Fibrosis (IPF) is a progressive scarring condition of the lung tissue with no known cause or cure. IPF remains a life-limiting disease with poor prognosis (median survival of 3-5 years from diagnosis1), so symptom relief is essential for improving quality of life. Cough is present in over 80% of patients with IPF2 and is often discordant with lung function measures. The exact mechanism of cough is unknown but there is evidence of altered cough neurophysiology and sensitisation3. Functional Magnetic Resonance Imaging (fMRI) has been used to investigate chronic cough in other conditions4, where neural pathways have been documented, but not in IPF. To permit the interpretation of future functional imaging studies into the neurophysiology of cough in this condition, it is important to understand the brain structure and baseline cerebrovascular function. Substantial underlying alterations in brain structure and physiology could modify the interpretation or interpretability of BOLD fMRI studies of cough. Therefore, before embarking on more complex testing of cough pathways we performed this study aiming to identify alterations in brain structure and cerebrovascular physiology in IPF as well as assessing tolerability of research MRI scans in this patient group.Methods
16 stable non-hypoxemic patients with IPF and 12 healthy controls matched for age and sex were assessed for demographic characteristics, disease severity and comorbidities. All participants underwent brain MRI performed on a clinical 3T scanner (Prisma MAGNETOM, Siemens Healthcare, Erlangen, Germany). A structural T1 weighted image (MPRAGE) for brain volume estimation was acquired. Participants underwent a resting pseudo-continuous arterial spin labelling (pCASL) sequence to assess resting grey matter perfusion (TR 4500ms, TE 11ms, slice thickness 5mm, GRAPPA acceleration factor 2, voxel size 3.4x3.4x5.0mm, post label delay (PLD) 2000ms, tag duration 1800ms) and a combined visual (visual checker-board) and motor task (paced finger tapping) in sequential 30 second blocks, acquiring both BOLD and ASL signals simultaneously (dual-excitation-pCASL (TE1 11ms, TR1 4200ms, TE2 30ms, TR2 900ms, slice thickness 7mm, and GRAPPA acceleration factor 2, voxel size 3.4x3.4x7mm, PLD 1800ms, tag duration 1800ms)). All analyses were performed using FSL (https://fsl.fmrib.ox.ac.uk/). SIENAX (FSL) was used to segment the brain5,6 and t-tests were performed to investigate differences between patients and controls in grey matter (GM) and white matter (WM) volumes. BASIL (FSL) was used to estimate baseline perfusion7; perfusion maps were then used to investigate differences between patients and controls with a voxel-wise approach8. FEAT (FSL) was used to detect vascular response to visual and motor tasks, as well as differences between groups during task execution9,10.Results
Patients were well matched for age, sex, smoking status and resting oxygen saturations (Table 1). No significant group differences in brain volumes were observed (grey matter volume (t(23.98)=1.09; p=0.29); white matter volume (t(20.74)=0.37; p=0.72)) but a weak trend towards reduced grey matter was noticed (Figure 1). IPF patients did show a significant reduction in grey matter perfusion at the whole brain level (t(22.53)=2.56; p=0.02)) compared to controls (Figure 2). Areas of significantly reduced perfusion in IPF were observed as localised to the precuneus cortex, cingulate gyrus and lingual gyrus (Figures 3 and 4). There were no significant differences in perfusion or BOLD signal between groups during the motor and visual tasks.Discussion / Conclusions
In this initial study of patients with IPF, gross alterations in brain volume were not observed but a significant reduction in grey matter perfusion was seen. Functional responses (BOLD and CBF signal changes) to simple visual stimuli and motor tasks were not different from controls. Localised reductions in cerebral perfusion were observed. These may arise from chronically reduced regional neuronal activity, regional grey matter atrophy or cerebrovascular differences in the posterior circulation. We speculate that the observed reductions in blood flow in the precuneus and nearby regions may arise from chronic reduction in the level of neuronal activity in the default mode network associated with the distracting influence of the condition, such as vigilance to symptoms including cough. Despite reduced blood flow, patients are able to meet cerebrovascular demands required for task execution with no significant difference compared to controls. Further investigation into the neural representation of such subjective symptoms is the subject of our future work.Acknowledgements
We would like to thank all the patients and healthy volunteers for participating in this research study and also staff members of the University Hospital of Llandough, Cardiff for their help with recruitment.References
1. S.D. Nathan, O.A. Shlobin, N. Weir, S. Ahmad, J.M. Kaldjob, E. Battle, M.J. Sheridan, R.M. du Bois, Long-term Course and Prognosis of Idiopathic Pulmonary Fibrosis in the New Millennium, Chest. 140 (2011) 221–229. doi:10.1378/CHEST.10-2572.
2. C.J. Ryerson, M. Abbritti, B. Ley, B.M. Elicker, K.D. Jones, H.R. Collard, Cough predicts prognosis in idiopathic pulmonary fibrosis, Respirology. 16 (2011) 969–975. doi:10.1111/j.1440-1843.2011.01996.x.
3. B.D.M. Hope-Gill, S. Hilldrup, C. Davies, R.P. Newton, N.K. Harrison, A Study of the Cough Reflex in Idiopathic Pulmonary Fibrosis, Am. J. Respir. Crit. Care Med. 168 (2003) 995–1002. doi:10.1164/rccm.200304-597OC.
4. A. Ando, D. Smallwood, M. McMahon, L. Irving, S.B. Mazzone, M.J. Farrell, Neural correlates of cough hypersensitivity in humans: Evidence for central sensitisation and dysfunctional inhibitory control, Thorax. 71 (2016) 323–329. doi:10.1136/thoraxjnl-2015-207425.
5. S.M. Smith, Y. Zhang, M. Jenkinson, J. Chen, P.M. Matthews, A. Federico, N. De Stefano, Accurate, robust, and automated longitudinal and cross-sectional brain change analysis., Neuroimage. 17 (2002) 479–89. http://www.ncbi.nlm.nih.gov/pubmed/12482100
6. S.M. Smith, M. Jenkinson, M.W. Woolrich, C.F. Beckmann, T.E.J. Behrens, H. Johansen-Berg, P.R. Bannister, M. De Luca, I. Drobnjak, D.E. Flitney, R.K. Niazy, J. Saunders, J. Vickers, Y. Zhang, N. De Stefano, J.M. Brady, P.M. Matthews, Advances in functional and structural MR image analysis and implementation as FSL, Neuroimage. 23 (2004) S208–S219. doi:10.1016/j.neuroimage.2004.07.051.
7. M.A. Chappell, A.R. Groves, B. Whitcher, M.W. Woolrich, Variational Bayesian Inference for a Nonlinear Forward Model, IEEE Trans. Signal Process. 57 (2009) 223–236. doi:10.1109/TSP.2008.2005752
8. A.M. Winkler, G.R. Ridgway, M.A. Webster, S.M. Smith, T.E. Nichols, Permutation inference for the general linear model, Neuroimage. 92 (2014) 381–397. doi:10.1016/j.neuroimage.2014.01.060.
9. M.W. Woolrich, B.D. Ripley, M. Brady, S.M. Smith, Temporal Autocorrelation in Univariate Linear Modeling of FMRI Data, Neuroimage. 14 (2001) 1370–1386. doi:10.1006/nimg.2001.0931.
10. M.W. Woolrich, T.E.J. Behrens, C.F. Beckmann, M. Jenkinson, S.M. Smith, Multilevel linear modelling for FMRI group analysis using Bayesian inference, Neuroimage. 21 (2004) 1732–1747. doi:10.1016/j.neuroimage.2003.12.023.
11. M.E. Charlson, P. Pompei, K.L. Ales, C.R. MacKenzie, A new method of classifying prognostic comorbidity in longitudinal studies: development and validation., J. Chronic Dis. 40 (1987) 373–83.
Figures

Table 1: demographics, brain volumes and perfusion in patients with IPF and controls. Data are n (%), mean (±SD)
FVC=forced vital capacity, TLCO=transfer factor of the lung for carbon monoxide, VAS=visual analogue scale 0-100mm, GM=grey matter, WM=white matter
+Charlson Comorbidity Index11
*pirfenidone or nintedanib
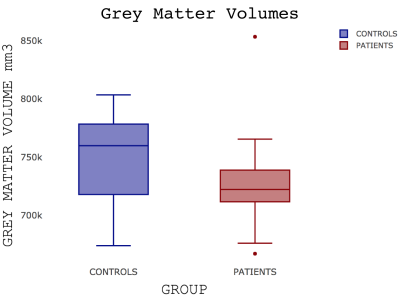
Figure 1: Boxplot showing grey matter volumes in controls and patients with IPF p=0.29
A line across the box depicts the median, the box indicates the 25% and 75% percentiles, whiskers represent the maximum and minimum values, circles represent outliers.
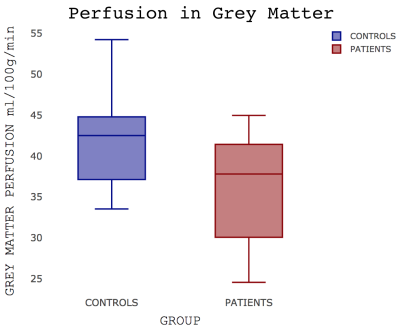
A line across the box depicts the median, the box indicates the 25% and 75% percentiles, whiskers represent the maximum and minimum values.
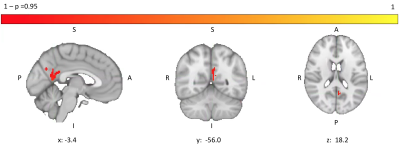
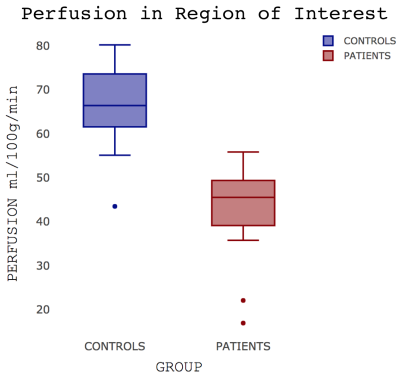
A line across the box depicts the median, the box indicates the 25% and 75% percentiles, whiskers represent the maximum and minimum values, circles represent outliers.