3824
Characterising the effect of flip angle in the acquisition of sub-millimetre resolution 3D GE-EPI at 7T1Maastricht Brain Imaging Centre (MBIC), Faculty of Psychology and Neuroscience, Maastricht University, Maastricht, Netherlands, 2Advanced MRI Technologies, Sebastopol, CA, United States, 3Helen Wills Neuroscience Institute, University of California, Berkeley, CA, United States
Synopsis
Sub-millimetre resolution is desirable for laminar-fMRI to reduce partial-voluming with neighbouring tissues. CSF signal fluctuations can be a confound in voxels that lie at the CSF-GM interface due to physiological processes such as activation. The 3D-EPI readout, used with sub-millimetre fMRI studies, suppresses the CSF signals (long-T1 vs. short-TR). However, the degree of suppression is dependent on the flip angle used. In this study, we investigate the signal contributions of different tissue compartments by systematically varying the flip angle and map the flip angle dependence of tSNR as a function of cortical depth at 0.8 mm isotropic resolution at 7T.
Introduction
One of the reasons laminar fMRI studies are carried out at sub-millimetre resolution is to reduce partial voluming of grey matter (GM) voxels with neighbouring tissues such as white matter (WM) and cerebrospinal fluid (CSF). During activation, changes in cerebral blood volume (CBV) due to vasodilation in tissue (GM swelling) or vessels on the cortical surface, could affect the volume fractions of regional CSF. This could affect the BOLD signal sampled at the uppermost layers at the CSF-GM boundary. It would hence be preferable to minimise the contribution from regional CSF. This especially applies to acquisitions in or near the physiological noise dominated regime, but also to fMRI acquisitions at a laminar resolution albeit at a lesser degree. One benefit of 3D EPI [1] as typically used for high-resolution fMRI is the relatively much stronger suppression of CSF (long T1 versus short TR) compared to 2D EPI acquisitions. This renders CSF nearly invisible in 3D EPI data and the degree of this CSF suppression depends on the flip angle (FA, α). There has been no systematic study into the effect of FA on the relative CSF and GM signal contributions, nor the temporal SNR (tSNR) of different tissue compartments. Here, we investigate the FA dependence of signal stability as a function of cortical depth, for the typical laminar resolution of 0.8mm isotropic voxels.Methods
Acquisition: All data were acquired on a Siemens Magnetom 7T (Siemens Healthineers) using a 32-channel head coil (Nova Medical). Two healthy volunteers (1male, 1female) participated in the study and all procedures were followed as per institutional ethical guidelines. The multi-flip-angle data were acquired at 0.8mm isotropic resolution using a modified 3D EPI sequence. The sequence allows the flip angle to be changed in a block-wise manner during the sequence run, thereby removing confounds due to the re-acquisition of parallel imaging ACS scans, system adjustments, and inter-run subject motion. This facilitates unbiased within-subject, within-scan impact of the choice flip angle. 80 TRs were acquired for each of six different flip angles (α=[5°,10°,15°,20°,15°,30°]) resulting in a total of 480 TRs. Imaging parameters were: TE=22ms, TR=3010ms, matrix size=202x202x32, BW=1126Hz/px, echo-spacing=1.01ms, GRAPPA factor=3, slab-selective excitation with BWTP=5.2. Total scan time per run was 24min for 480 volumes. The acquisition slab was positioned to cover the motor and somatosensory cortices with A>P phase-encoding. An MP2RAGE [2] was acquired at 0.8mm isotropic resolution as anatomical reference.Analysis: The multi-flip-angle data were sorted by flip angle and two transition volumes at the beginning and end of each flip angle cycle were discarded (i.e. total 76/80 TRs per flip angle used for tSNR calculation). The data were then motion-corrected and distortion-corrected using ANTs [3]. tSNR maps were then calculated. The MP2RAGE T1 map was coregistered to the mean of the time-series, and tissue segmentation was carried out using FSL-FAST. A vein mask was obtained from the mean EPI image acquired with α=15° by thresholding to very low intensities, and these voxels were excluded from the CSF mask that is used to calculate the mean and tSNR measures. For laminar fMRI the objective is to maximise the GM tSNR. To this end, we computed the depth-dependent tSNR, restricting the region-of-interest to approximately the hand region of the motor cortex using CBS-Tools [4].
Results and discussion
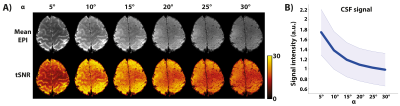
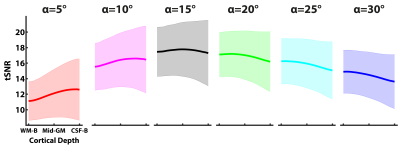
Identically windowed single-subject mean EPI and tSNR maps at the six different flip angles are shown in Figure 1A. The mean EPI images show the expected effect of CSF signal being increasingly suppressed with increasing flip angle. This suppression of the CSF signal is more clearly illustrated in Figure 1B. To optimise the GM tSNR, the flip angle used in high-resolution 3D GE-EPI studies is the Ernst angle ([5], in our case ~α=15°). Figure 2 shows that the tSNR is maximal at the Ernst angle, consistent with theoretical predictions. Furthermore, the GM tSNR laminar profile at α=15° is relatively unvarying across cortical depths, having almost zero slope. This indicates there is no baseline bias from the acquisition with regard to any observed changes in slope during activation [6-8]. For flip angles shorter than the 15°, we observe GM tSNR increasing towards the cortical surface. On the other hand, for flip angles higher than 15°, we observed GM tSNR decrease towards the cortical surface. This signal behaviour at the cortical surface i.e. CSF boundary is consistent with CSF suppression in 3D EPI as illustrated Figure 1B. These findings are in line with theoretical expectations and are particularly reassuring for laminar fMRI studies using 3D GE-EPI. Finally, while exciting at the Ernst angle is ideal, our preliminary findings indicate that erring on the side of slightly higher flip angles than lower in the ROIs can be preferable and there is headroom available without any penalty given the SAR efficiency of the 3D EPI readout. Next steps are to carry out a follow-up study using a stimulus paradigm and investigate the effects of different flip angles on the BOLD activation profile across cortical depths, especially at the CSF-GM boundary.Acknowledgements
SK and BAP are funded by the NIH R01 MH111444/MH/NIMH (PI: DAF), DK and BAP are funded by NWO VIDI 016.178.052, and LH is funded by NWO VENI 016.198.032.References
[1] Poser et al., 2010 (doi: 10.1016/j.neuroimage.2010.01.108), [2] Marques et al., 2010 (doi: 10.1016/j.neuroimage.2009.10.002), [3] Avants et al., 2011 (doi: 10.1016/j.neuroimage.2010.09.025), [4] Bazin et al., 2014 (doi: 10.1016/j.neuroimage.2013.03.077), [5] Ernst and Anderson, 1966 (doi: 10.1063/1.1719961), [6] Polimeni et al., 2010 (doi: 10.1016/j.neuroimage.2010.05.005), [7] Koopmans et al., 2011 (doi: 10.1016/j.neuroimage.2011.02.042), [8] Kashyap et al., 2018 (doi: 10.1016/j.neuroimage.2017.05.022)
Figures