3819
Simultaneous functional and anatomical high-resolution imaging using BISEPI at 7T
Guoxiang Liu1,2, Adnan Shah1,2, and Takashi Ueguchi1,2
1Brain Function Analysis and Imaging Lab, CiNet, NICT, Osaka, Japan, 2Graduate School of Frontier Biosciences, Osaka University, Osaka, Japan
1Brain Function Analysis and Imaging Lab, CiNet, NICT, Osaka, Japan, 2Graduate School of Frontier Biosciences, Osaka University, Osaka, Japan
Synopsis
A novel method for simultaneous functional and anatomical imaging is presented for high-resolution fMRI at 7T. An RF inversion pulse is used before two EPI acquisition trains where functional scans are acquired alternating between shorter and longer inversion times (TIs). Post-processing division of the shorter TI functional scan with the longer TI functional scan allows improved localization of brain activity in high-resolution fMRI at 7T. The proposed method allows simultaneous imaging of brain function and anatomy as the grey matter tissues can easily be segmented out from the resulting functional images, ensuring their perfect alignment required for detecting functional activity.
Introduction:
Discriminating BOLD responses at the level of cortical layers require isotropic submillimeter resolution fMRI, which is possible at ultra-high-field (UHF, 7T or higher) scanners. However, functional imaging at 7T suffers from large image distortions resulting in a strong misregistration between function and anatomy much exacerbated for submillimeter-resolution imaging. In ISMRM 2018, we proposed an imaging and reconstruction method referred to as Block-Interleaved Segmented EPI (BISEPI) for reconstructing fMRI volumes using discontinuous segmented k-space data [1]. BISEPI method allows us to perform isotropic submillimeter resolution BOLD fMRI while simultaneously preserving the hemodynamic response. In this work, we report a novel imaging technique based on BISEPI that allows us to investigate sub-millimeter-level brain activity without the need of an additional anatomical scan. An RF inversion pulse is used before two EPI acquisition trains where functional scans are simultaneously acquired alternating between shorter and longer inversion times (TIs). Post-processing division of the shorter TI scan with the longer TI scan allows improved localization of brain activity in high-resolution fMRI. Moreover, the proposed method improves the specificity of detected brain activity by suppressing contribution from arteries and veins. The proposed method avoids functional to structural transformation and spatial smoothing, giving us an opportunity to investigate brain activity dynamics at the columnar or even layer level spatial resolution. Our results showed that the proposed method can be considered as a choice to perform voxel-wise high-resolution layer fMRI studies at 7T, as the functional and anatomical information can simultaneously be obtained from the functional scan.Materials & Methods:
In the proposed sequence, an inversion pulse is applied before two EPI acquisition trains as shown in Figure 1. A human brain was scanned using this sequence on a Siemens MAGNETOM 7T scanner with a 32-channel phased array head coil (Nova Medical, MA, USA) to obtain two EPI series data (48 volumes each) at a spatial resolution of 0.6×0.6×2.0 mm3. Acquisition parameters for functional/structural scans were as follows: TR = 3000 ms, TE = 23 ms, flip angle (FA) = 90°, # segments = 6, # shots in block = 16, PF = 6/8. A bilateral finger tapping task was performed using BISEPI design [1] with ON and OFF for 12 s each. Six slices were scanned alternating between shorter and longer groups of inversion times (TIs). TI1: 850ms to 1200ms, and TI2: 2350ms to 2700ms. Division of EPI1 (shorter TIs) by EPI2 (longer TIs) in each TR was calculated to build a new time series called DVI=EPI1/EPI2. Three series (EPI1, EPI2, DVI) were analyzed in BrainVoyager QX (Version 2.8.4.2645 Brain Innovation, Maastricht, The Netherlands). The following pre-statistics processing was applied; interscan slice time correction, high-pass filtering using a general linear model (GLM) Fourier bases set of 2 sine/cosines as well as temporal Gaussian smoothing with a full width at half maximum (FWHM) kernel of 2 data points. No spatial smoothing was performed. GLM analysis was applied with stimulation ON/OFF as a binary regression variable.Results and Discussion:
Figure 2 shows the same slice from EPI1, EPI2, and DVI in our experiment, and the grey mater tissues segmented from DVI using 2D k-means algorithm [2]. Figure 3 compares the brain activity detection results of each series for two different slices and the different TIs. EPI2 shows similar normal BOLD response with some veins suppressed by the inversion pulse. BOLD-like response detected in EPI1 series is more likely coming from arteries, because uninverted blood should enter the microvasculature later than 1.4s after the inversion pulse applied [3] , while the TI1 in this experiment is shorter than 1.2s. Negative BOLD-like response in DVI series is observed to be located in grey matter area, showing the possibility that the effect of arteries was canceled by the division. Another possible component of the negative BOLD-like response in DVI series is VASO [4], even though we could not detect VASO response directly from EPI1 series. Although signal inhomogeneity in both EPI1 and EPI2 is serious, but the division processing of EPI1 and EPI2 removed most of the signal inhomogeneity caused by varying tissue T2*, B1 and even instability of the scanner, resulting in a pure T1-weighted image, which can be segmented into grey/white matter tissues for next step analysis.Acknowledgements
This study was supported in part by Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research “KAKENHI” (Grant Numbers JP26282223 and JP26350471 and JP19K08244).References
[1] Liu G, Shah A, Ueguchi T. Block-Interleaved Segmented EPI for voxel-wise high-resolution fMRI studies at 7T. Proc. Intl. Soc. Mag. Reson. Med. 26 (2018) 5450. [2] MacQueen JB. Some Methods for Classification and Analysis of Multivariate Observations. In: Cam, L. M. L., Neyman, J. (Eds.), Proceedings of 5-th Berkeley Symposium on Mathematical Statistics and Probability. Vol. 1. University of California, Berkeley, Berkeley, USA; 1967; 1:281–297. [3] Chen Y, Wang DJJ, Detre JA. Comparison of arterial transit times estimated using arterial spin labeling. Magnetic Resonance Materials in Physics, Biology and Medicine, 2012; 25:135–144. [4] Lu H and van Zijl PC. A review of the development of Vascular-Space-Occupancy (VASO) fMRI. Neuroimage. 2012 Aug 15;62(2):736-42.Figures
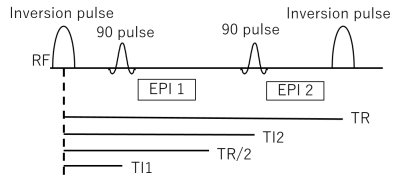
Figure 1: An inversion pulse is applied before
two EPI acquisition trains.
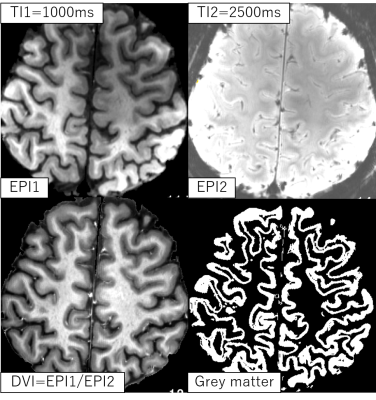
Figure 2: A single slice from the EPI1,
EPI2, DVI, and the corresponding grey mater tissues segmented from
DVI using 2D k-means algorithm.
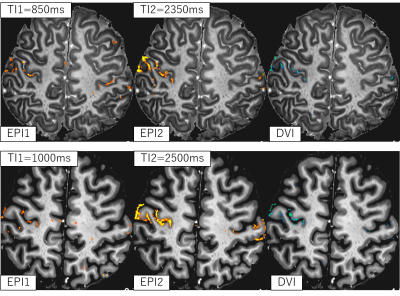
Figure 3: Brain
activity detection results of each series for two different slices and the different
TIs overlaid on DVI image.