3818
Mouse fMRI of visual stimulation in awake vs. anesthetized condition
THI NGOC ANH DINH1 and Seong Gi Kim1
1Department of Biomedical Engineering, Sungkyunkwan University, Center for Neuroscience Imaging Research, Institute for Basic Science (IBS), Suwon-si, Korea, Republic of
1Department of Biomedical Engineering, Sungkyunkwan University, Center for Neuroscience Imaging Research, Institute for Basic Science (IBS), Suwon-si, Korea, Republic of
Synopsis
Mouse fMRI has been increasingly used in the MRI community. Here, we developed an awake mouse fMRI protocol and compared BOLD fMRI responses responding to visual stimulation of 5 Hz and 10 Hz, obtained during awake and anesthetized conditions. Our 9.4-T BOLD-fMRI showed activities in all visual areas including LGN, superior colliculus, and V1, and can be well-explained by electrophysiology literature.
Introduction
Mouse fMRI has been increasingly interested in the MRI community due to abundant transgenic models and available animal-dedicated MRI scanners and has been successfully conducted under anesthesia1,2,3. In our fMRI study (ISMRM 2019, program #362), ketamine/xylazine mixture (glutamate receptor antagonist) was successfully adopted for visual stimulation. However, anesthetic agents affect neural activity and hemodynamic responses, consequently, the choice of anesthesia will make a great impact on fMRI responses1,2. Therefore, it’s ideal to perform experiments under an awake condition. Our specific goals are i) to develop an awake mouse fMRI protocol, and ii) to compare fMRI responses obtained during wakefulness vs. ketamine/xylazine anesthesia.Methods
Animal PreparationFourteen male C57BL/6N mice (8-10 weeks old) were used with approval; seven for awake fMRI, two for corticosterone measurements during habituation and five for anesthetized fMRI. MR-compatible head fixation and body restraint apparatus were designed for minimizing head and body motions. The head fixation (Fig. 1) was bonded to the dorsal skull after skin removal under ketamine/xylazine anesthesia. After 7-day recovery, mice were habituated for 10 days: 7 days in the mock scanner with 110–120dB EPI sound and 3 remaining days in the 9.4T scanner. Seven mice were performed for 2-hour fMRI scanning in wakefulness. In another two mice, 40µl tail vein blood was collected from each mouse at 4 days before, immediately after the 1st day, 5th day and 10th day of habituation. For anesthetized fMRI, ketamine/xylazine3 was used.
Functional Imaging Data Acquisition
fMRI was conducted on 9.4T/30cm Bruker scanner using single-shot GE-EPI sequence with TR/TE=1000/20ms, flip-angle=50°, spatial resolution=156×156×500μm3, and 9 coronal contiguous slices without gap. For visual stimulation, two 0.5mm diameter optic fibers were placed bilaterally 2cm away from both eyes of the animal, and connected to white cold LED driver (Thorlabs DC2000). Stimulus parameters were illuminance of ~10lux, pulse duration of 10ms, and two different frequencies of 5Hz and 10Hz. Each fMRI trial consisted of 30s offs–10s on–40s off–10s on–30s off (Fig. 1). Six to eight trials were obtained for each frequency.
Data Analysis
Data were processed with Matlab and AFNI. Frame-wise displacement (FD) was calculated to evaluate animal motions before preprocessing. If the FD value exceeded the threshold of 1 voxel size or changed synchronously with the stimulus period, the trial was excluded from further analysis. The preprocessing included slice timing correction, motion correction, temporal detrending, temporal normalization from baseline and trial averaging. Then data were normalized to an EPI template, standard GLM analysis was applied to identify significant BOLD responses. Regions of interest (ROI) were defined on EPI images based on Allen Mouse Brain Atlas (Fig. 1), which were the dorsal lateral geniculate nucleus (LGd), superior colliculus (SCs), lateral posterior nucleus of the thalamus (LP), primary visual cortex (V1), and higher-order visual area (V2). The group activation maps were generated using a one-sample t-test with 0.2mm FWHM Gaussian kernel spatial smoothing (p<0.05; FWE corrected).
Results
Since awake fMRI induces stress, habituation is critical for reducing stress. Corticosterone levels were ~10ng/ml before started, 30ng/ml on the 5th day and 15ng/ml after the 10th day of habituation (n=2), suggesting the stress level is reduced by habituation, but still modest. Among seven mice, one was excluded because of large motion. 77% of trials (65/84 total trials in 6 animals) passed the motion criteria. Fig. 2A shows the group-level BOLD-fMRI activation maps of 5Hz stimulus for awake and anesthetized mice. Most ROIs (Fig. 1) were robustly activated in both conditions except V1. Time courses from ROIs show detailed temporal characteristics (Fig. 2B). LGd, LP, and SCs for both conditions had similar responses. Unlike the anesthetized, the response in V1 for the wakefulness increased sharply, followed by a quick decay, leading to negative BOLD change during the stimulation period. When stimulus frequency increased to 10Hz (Fig. 2C), activities in awake mice were increased in subcortical areas compared to the anesthetized, but fully suppressed in cortical areas. This observation in V1 is quite unexpected. BOLD time courses of all ROIs were normalized to compare dynamic response between two conditions (Fig. 3). The peak response of awake mice was apparently 1–3s earlier in all regions compared to the anesthetized (Fig. 3).Discussion & Conclusion
We have successfully developed an awake fMRI protocol by designing the restraint apparatus and habituation steps. For both awake and anesthetized conditions, BOLD-fMRI activities were observed in all visual pathway-related areas. The most interesting observation is V1 activity; under anesthesia, the BOLD activity is prolonged and spreads a larger area, while the awake BOLD response is biphasic/negative. This observation can be explained by electrophysical studies4,5. During wakefulness, inhibition is much stronger than excitation and has extremely broad spatial selectivity, resulting in a brief and spatially selective BOLD response. Under the wakefulness, the temporal frequency tuning of LGd, LP and SCs were shifted toward higher frequency, while V1 had no significant differences. Since our fMRI observations concur with the published electrophysiology data4,5,6,7,8, our awake fMRI system is well-acceptable for neuroscience research. Based on this preliminary result, further studies will be conducted with the awake mouse model for filling the gap between macroscopic fMRI and microscopic electrophysiology studies.Acknowledgements
This work was supported by IBS-R015-D1.References
- Schlegel F, Schroeter A, Rudin M. The hemodynamic response to somatosensory stimulation in mice depends on the anesthetic used: implications on analysis of mouse fMRI data. Neuroimage. 2015 Aug 1; 116:40-9.
- Petrinovic MM, Hankov G, Schroeter A, Bruns A, Rudin M, Von Kienlin M, Künnecke B, Mueggler T. A novel anesthesia regime enables neurofunctional studies and imaging genetics across mouse strains. Scientific reports. 2016 Apr 15; 6:24523.
- Shim HJ, Jung WB, Schlegel F, Lee J, Kim S, Lee J, Kim SG. Mouse fMRI under ketamine and xylazine anesthesia: Robust contralateral somatosensory cortex activation in response to forepaw stimulation. Neuroimage. 2018 Aug 15; 177:30-44.
- Haider B, Häusser M, Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature. 2013 Jan;493(7430):97.
- Vaiceliunaite A, Erisken S, Franzen F, Katzner S, Busse L. Spatial integration in mouse primary visual cortex. Journal of neurophysiology. 2013 May 29;110(4):964-72
- Durand S, Iyer R, Mizuseki K, de Vries S, Mihalas S, Reid RC. A comparison of visual response properties in the lateral geniculate nucleus and primary visual cortex of awake and anesthetized mice. Journal of Neuroscience. 2016 Nov 30;36(48):12144-56.
- De Franceschi G, Solomon SG. Visual response properties of neurons in the superficial layers of the superior colliculus of awake mouse. The Journal of physiology. 2018 Dec;596(24):6307-32.
- Gao YR, Ma Y, Zhang Q, Winder AT, Liang Z, Antinori L, Drew PJ, Zhang N. Time to wake up: Studying neurovascular coupling and brain-wide circuit function in the un-anesthetized animal. Neuroimage. 2017 Jun 1; 153:382-98.
Figures
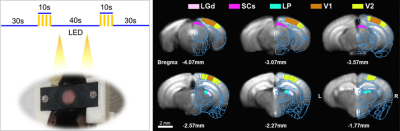
Experimental setup and ROIs. Left, visual
stimulation paradigm and the head bar for awake fMRI. Right, ROIs on EPI
template based on Allen Mouse Brain Atlas, dorsal lateral geniculate nucleus
(LGd), superior colliculus (SCs), lateral posterior nucleus of the thalamus (LP), primary
visual area (V1), and higher-order visual area (V2).
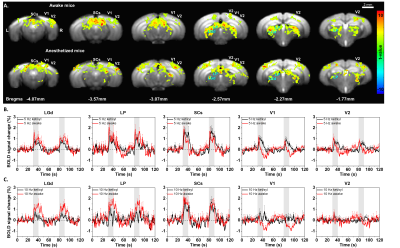
Functional activation maps and BOLD time courses
responding to visual stimulation for awake and anesthetized conditions. (A) Group
activation maps responding to 5Hz stimulus of 6 awake and 5 anesthetized mice (p
< 0.05, FEW corrected). (B-C) Averaged BOLD time courses of all visual ROIs
of awake (red) and anesthetized (black) mice at 5Hz (B) and 10 Hz stimuli (C). Error
bar: SEM.
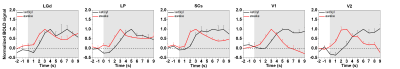
Time to peak of all visual
ROIs between two conditions: awake (red) and anesthetized (black). Averaged,
normalized BOLD time courses of each ROI responding to 5Hz stimulation were
obtained for each condition. Only a 2-s pre-stimulus onset period and an
initial 10-s post-stimulus onset period were plotted. Error bar: SEM.