3813
Optimized on-the-fly reconstruction for combined T2*-weighted imaging of the human brain and cervical spinal cord1Department of Systems Neuroscience, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Synopsis
Cortico-spinal functional magnetic resonance imaging (fMRI) covering slices in the brain and cervical spinal cord usually involves specific geometry (field-of-view, voxel size) and timing parameters (bandwidth) for the two volumes. This requires a time-consuming and cumbersome retrospective reconstruction after the experiment because the standard program cannot handle the different parameter settings properly, e.g., for regridding of ramp-sampled data or correction of distortions induced by Maxwell terms. Here, a reconstruction program is presented that performs an optimized reconstruction on-the-fly including the correction of receive-coil inhomogeneities without the need of a retrospective reconstruction. It could help the applicability of cortico-spinal fMRI.
Introduction
Combined functional magnetic resonance imaging (fMRI) based on blood-oxygenation-level-dependent (BOLD) contrast1 of the brain and cervical spinal cord2 allows to assess the functional connectivity between the two regions, e.g. in pain processing.3 Ideally, dedicated in-plane resolutions, slice thicknesses, receiver bandwidth, and timing parameters are used for each of the target volumes to have the best settings considering its specific needs. However, standard MR systems usually cannot handle different values for these parameters which means that some image reconstruction steps like regridding of ramp samples and the correction of Maxwell terms will only work properly for one of the two volumes on-the-fly. So far, a work-around involved a second, retrospective reconstruction of corresponding fMRI data sets with the parameters set to that of the volume that has not been reconstructed properly on-the-fly during the experiment. However, this is not only cumbersome and error-prone but also time consuming and blocks the MR system for significant time (typically more than 20min) making cortico-spinal fMRI less feasible in practice.Methods
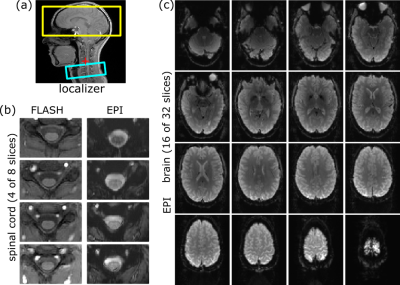
Experiments were performed on a 3T whole-body MR-system (PrismaFit, Siemens Healthineers) using a 64-channel head-neck coil in cylindrical water phantom and in vivo. Healthy volunteers were investigated after their informed consent was obtained. Echo-Planar Imaging (EPI) acquisitions of the brain sub-volume (32 slices) were performed using a square FOV of 256mm, an in-plane resolution of 2.0x2.0mm2, and a slice thickness of 2mm with a 1mm gap; in the spinal cord sub-volume (8 slices), the FOV is 128mm, the in-plane resolution 1.0x1.0mm2, and the slice thickness 5mm without a gap (Fig. 1). Both volumes were acquired with a TE of 30ms yielding a minimum TR of 2696ms. A dynamic update of the frequency and first order shim values was performed between the two volumes as described in the previous study.2 The isocenter was positioned close to the spinal cord volume (see Fig. 1a) which improved the overall shim performance but caused significant distortions due to the Maxwell terms4 in the brain volume.The image reconstruction program was modified to distinguish slices from the brain and spinal cord volume and consider the corresponding geometry and timing parameters for the reconstruction steps that depend on these parameters, in particular regridding of ramp samples, correction of distortions induced by concomitant field gradients (“Maxwell terms”), and correction of coil sensitivity inhomogeneities. The required parameters can be derived from the acquisition protocol or are passed to the reconstruction program during the acquisition. Images obtained with the modified program (“optimized”) on-the-fly are compared to those obtained conventionally on-the-fly (“conventional”, reconstruction parameters of the spinal cord volume) and with a subsequent retrospective reconstruction (“retrospective”, reconstruction parameters of the brain volume). Only results for the brain volume are presented because the reconstruction of the spinal cord volume is not affected and performed optimally with all reconstructions involved. The same data sets were used for the comparisons, i.e. any difference observed must be due to the different reconstruction processes.
Results and Conclusion
Figure 2 demonstrates the effect of the different timing parameters on the regridding of ramp-sampled data for the brain volume. Conventionally reconstructed images suffer from ringing-like artifacts that previously required a retrospective reconstruction but now are also avoided with the modified program.The effect of Maxwell terms and its correction without and with proper reconstruction parameters is shown in Fig. 3. A significant shearing of the brain in the EPI images compared to the anatomical reference is observed with the conventional reconstruction that is corrected with the modified program, providing a much better representation of the brain’s geometry. Residual deviations from the anatomical outline are due to geometric distortions induced by inhomogeneities of the magnetic flux density close to air cavities and cannot be corrected in this reconstruction step.
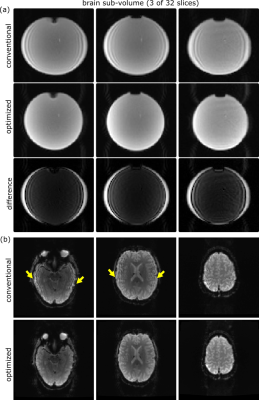
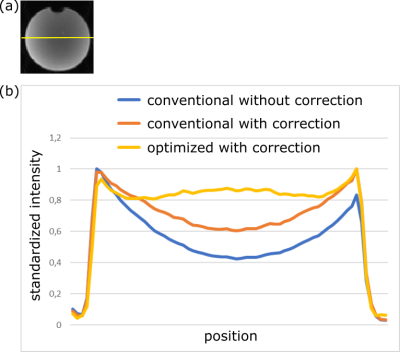
The influence of the geometric parameters on the correction of the receive coil sensitivity inhomogeneities is presented in Fig. 4 in a phantom. The conventional on-the-fly reconstruction does not consider the specific geometry of the brain slices and does not perform a proper correction. In contrast, the modified program is able to provide a good correction with a very homogenous signal amplitude across the phantom.
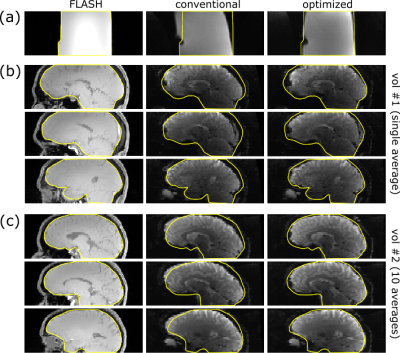
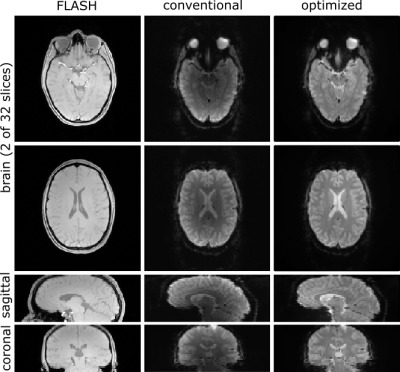
Figure 5 shows brain EPI images obtained with the retrospective and the modified on-the-fly reconstruction. The modified program performs as well as the retrospective reconstruction with respect to regridding and correction of Maxwell terms but applies a proper correction of the coil sensitivities yielding a more homogeneous signal intensity distribution across the brain.
In conclusion, the modified reconstruction program performs an optimal reconstruction of corticospinal fMRI data without the need of a cumbersome and time consuming retrospective reconstruction. Thus, it could help to facilitate the applicability of cortico-spinal fMRI significantly.
Acknowledgements
This research was supported by the German Research Foundation DFG (SFB936/A6).References
1. Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990: 87(24):9868-9872.
2. Finsterbusch J, Sprenger C, Buchel C. Combined T2*-weighted measurements of the human brain and cervical spinal cord with a dynamic shim update. NeuroImage.2013;79:153-161.
3. Tinnermann A, Geuter S, Sprenger C, Finsterbusch J, Büchel C. Interactions between brain and spinal cord mediate value effects in nocebo hyperalgesia. Science.2017;358:105-108.
4. Bernstein MA, Zhou XH, Polzin JA, et al. Concomitant gradient terms in phase contrast MR: Analysis and correction. Magnetic Resonance in Medicine.1998;39(2): 300-308.
Figures