3807
Cross-Field Strength and Cross-Vendor Reliability of Quantitative MR-based Breast Density (MagDensity)1Biomedical Engineering, Stony Brook University, Stony Brook, NY, United States, 2Diagnostic Radiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, Hong Kong, 3Radiology, Stony Brook University, Stony Brook, NY, United States, 4Psychiatry, Stony Brook University, Stony Brook, NY, United States
Synopsis
Quantitative measurement of breast density is important for personalized risk assessment and frequent, longitudinal monitoring of breast density modifying drugs. Current standard of care for assessing breast density is qualitative assessment of mammogram, which involves ionizing radiation and breast compression. MRI-derived breast density (MagDensity) using fat-water decomposition has the potential to provide a sensitive and useful tool for clinicians. This study aims to prospectively determine the reliability and reproducibility of this measure across different vendors, scanner models and field strengths
Introduction
Women with dense breasts have up to a 6-fold increased risk for developing breast cancer[1, 2]. It also has been shown to be modifiable risk factor, with several clinical trials aimed at decreasing breast density[3-5]. Current standard for measuring breast density is a qualitative assignment to one of four categories (fatty, scattered fibroglandular, heterogeneously dense, extremely dense) by the radiologist based on mammogram. Quantitative assessment recently gained substantial interest because it could help clinicians to better estimate risk, aid in implementation of personalized medicine and facilitate more sensitive, frequent longitudinal monitoring of breast density modifying drugs. Fat-water decomposition MRI has the potential to measure breast density quantitatively using MRI without the use of ionizing radiation[6]. This study aims to prospectively determine the reliability and reproducibility of this quantitative measure across different vendors, scanner models and field strength.Methods
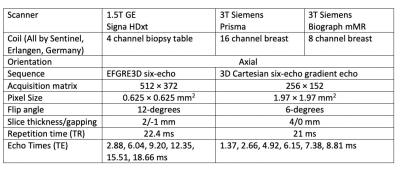
In this ongoing study, ten volunteers have been prospectively recruited and scanned on three different scanners (1.5T GE Signa HDxt, 3T Siemens Prisma and 3T Siemens Biograph mMR) the same day; data from 2 subjects were excluded due to significant motion artifact. A six-echo gradient echo acquisition was performed on each scanner with different parameters (see Figure 1).A graph-cut algorithm was used to separate signal from water and from fat, while correcting for R2* decay, field inhomogeneity, eddy current, and fat-water signal intensity bias. Two quantification metrics were compared, namely Fra90 (ratio of breast voxels with < 90% fat fraction, which is a threshold-based approach) and FraWater (amount of water versus fat in terms of volume); each of these measures were then converted to MRI density based on correlation relationships between the measure and mammographic density using a conversion function and referred to as DensityFra90 and MagDensity, respectively[6]. Intraclass correlation coefficient analysis and mean difference was assessed to determine the absolute agreement between these measured values.Results
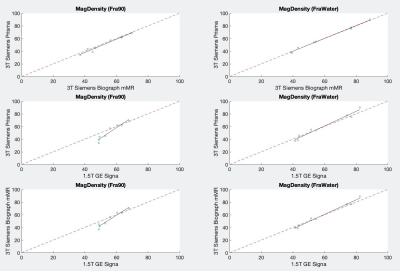
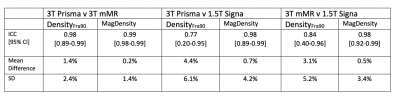
Figure 2 shows a representative example of the fat ratio maps across the three different scanners after correcting for fat-water signal intensity bias[6]. Results are summarized in Figure 3 and Figure 4. Figure 3 shows the pair-wise agreement between measured values of the different scanners. MagDensity has a higher degree of agreement than DensityFra90 as indicated by being nearer to the identity line. Both MagDensity and DensityFra90 exhibited high reliability between the two Siemens 3T scanners (ICC 0.99, 0.98 respectively). However, the reliability across field strength was markedly higher for MagDensity (ICC: 0.98) compared to DensityFra90(ICC: 0.77-0.84). Similar behavior is also seen in mean difference, which is larger for DensityFra90 compared to MagDensity (1.4% v.s. 0.2% same field strength, 3.1-4.4% v.s. 0.5-0.7% across field strength, respectively).Discussion
Accurate and precise quantification of breast density using MRI will allow for personalized risk assessment and sensitive, frequent longitudinal monitoring of breast density modifying drugs without the use of ionizing radiation. Current standard for assessing breast density using mammography leads to high intra- and inter-reader making it unsuitable to observe subtle, small changes in breast density; variability can be introduced by different degree of breast compression, variation of tissue inclusion near chest wall and/or different x-ray calibration parameters[7]. For clinical adoption of this breast density measure, it needs to be robust and reliable across different scanners, field strengths and vendors. Our study showed quantitative MR-based breast density measure to be in good agreement across three different scanners. Specifically, MagDensity is a more reliable measure compared to DensityFra90. One limitation of the study is the lack of low-density subjects, we are in the process of recruiting more these subjects.Conclusion
Quantitative measure of MRI breast density (MagDensity) is reproducible across different scanners and field strengths.Acknowledgements
This work is in part funded by National Institutes of Health (R03CA223052), Walk-for-Beauty Foundation and Carol M. Baldwin Breast Cancer Research Foundation.References
1. Freer, P.E., Mammographic breast density: impact on breast cancer risk and implications for screening.Radiographics, 2015. 35(2): p. 302-15.
2. McCormack, V.A. and I.D.S. Silva, Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis.Cancer Epidemiology Biomarkers & Prevention, 2006. 15(6): p. 1159-1169.
3. Cuzick, J., et al., Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study.J Natl Cancer Inst, 2011. 103(9): p. 744-52.
4. Thomson, C.A., et al., A randomized, placebo-controlled trial of diindolylmethane for breast cancer biomarker modulation in patients taking tamoxifen.Breast Cancer Res Treat, 2017. 165(1): p. 97-107.
5. Nyante, S.J., et al., Longitudinal Change in Mammographic Density among ER-Positive Breast Cancer Patients Using Tamoxifen.Cancer Epidemiol Biomarkers Prev, 2016. 25(1): p. 212-6.
6. Ding, J., et al., Reproducible automated breast density measure with no ionizing radiation using fat-water decomposition MRI.J Magn Reson Imaging, 2018. 48(4): p. 971-981.
7. Kerlikowske, K., et al., Variability and accuracy in mammographic interpretation using the American College of Radiology Breast Imaging Reporting and Data System.J Natl Cancer Inst, 1998. 90(23): p. 1801-9.
Figures