3806
CNN-based synthesis of T1, T2 and PD parametric maps of the brain with a minimal input feeding1Laboratorio de Procesado de Imagen, Universidad de Valladolid, Valladolid, Spain
Synopsis
Parametric MR maps (T1, T2 and PD) not only play a key role in quantitative imaging but they also have the capability of synthesizing any modality. However, their direct acquisition is hardly used in practice due to the need of lengthy relaxometry protocols. Synthetic MRI is a surrogate; however, no approach has been described to synthesize these maps out of a small number of customary sequences. In this work we synthesize T1, T2 and PD maps out of a T1- and a T2-weighted image using a CNN trained only with synthetic data. Our approach yields realistic maps from real data.
Purpose
Synthetic MRI has received significant attention during the past decade due to its ability to generate realistic MR images. The synthesized images obtained with this novel technique may improve diagnostic reliability, allow data augmentation to develop deep-learning approaches and facilitate physicians training1. Recently, various synthetic MRI approaches have been proposed that learn the mapping between different pairs of images2-4, although most of them are tailored for specific applications where, given some inputs, new image modalities can be synthesized. For example, in Ref4 they synthesize T1-weighted images from T2-weighted images. However, despite their usefulness, these approaches do not take advantage of the whole potential of MRI, particularly of quantitative parametric maps (i.e., T1, T2 and PD maps) which would allow the synthesis of any image modality by means of simulation. Further, even though quantitative maps play an important role in tissue and disease characterization5,6, they are usually not obtained in the clinical routine because of the time needed to acquire them and because most methods only provide information of a single parameter7-9. To overcome these limitations, MR fingerprinting10 is able to estimate multiple parametric maps within a short acquisition time, albeit the estimated maps are slightly different to those obtained with traditional techniques. Thus, the purpose of this work is to synthesize realistic T1, T2 and PD quantitative parametric maps from only two input images (i.e., T1- and T2-weighted images) utilizing a supervised deep convolutional neural network3 (CNN).Methods
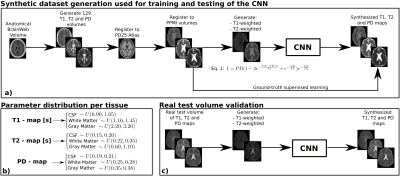
Generation of a synthetic dataset: Starting from the BrainWeb11 synthetic brain anatomical volume, we simulated 129 synthetic volumes as follows: 1) We created 129 different T1, T2, and PD maps by adding variability with uniformly distributed random values to the white matter, grey matter and cerebrospinal fluid (CSF) of each map (see Figure 1 for details). Then, additive Gaussian noise was added to each volume with distribution $$$N(\mu=0,\sigma=0.01)$$$. 2) To introduce spatial variability across volumes (i.e., brains with different anatomical features), we registered the T1, T2 and PD maps to the PD25 atlas12 and then registered each set of T1, T2, and PD maps to 129 different T1 volumes from the public PPMI database13 with 1mm3 resolution. Registrations were performed following the FSL14 pipeline. 3) For each set of ground-truth T1, T2 and PD synthetic maps, we theoretically simulated15 T1- and T2-weighted images of spin-echo acquisitions with TR=500ms, and TE=15ms, and TR=2200ms and TE=100ms, for the T1- and T2-weighted images, respectively.Neural network training with the synthetic dataset: We modified the CNN employed in Ref3 to synthesize T1, T2 and PD maps from a T1- and a T2-weighted image. These 2D weighted images were input to two encoders and fused to a latent representation in order to obtain the three parametric maps through three decoders. The model was trained using Adam optimizer with a batch size of 8 images and used early stopping to avoid overfitting. From the 129 synthetic volumes with two MR weighted images, we used 89 for training (22,784 2D images), 26 for validation (6,656 2D images), and 14 for test (3,584 2D images).
Validation on a real test volume: Real T1, T2 and PD maps of a healthy volunteer obtained at a 3T MRI (Philips, Best, The Netherlands) were downloaded from the MR simulator of Ref1. The maps were registered to the PPMI space using the previously described pipeline. Then, we generated spin-echo T1- and T2-weighted images with the same time parameters than the synthetic dataset.
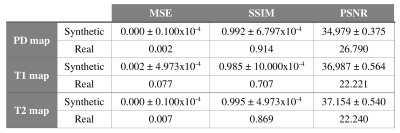
Finally, for each set of skull-stripped T1- and T2-weighted images, the CNN provided the synthesized T1, T2, and PD maps (see Figure 1) and the following metrics were used to evaluate its performance: mean-squared-error3, structural-similarity-error index3, and peak signal-to-noise-ratio3.
Results
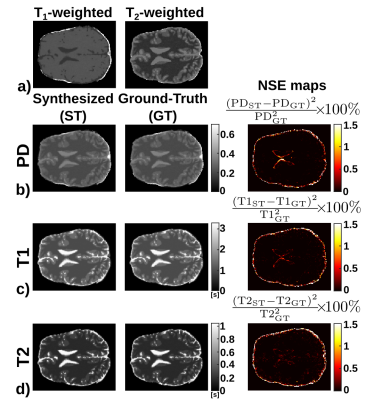
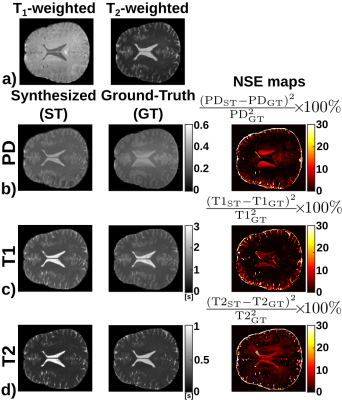
Figure 2 shows the T1, T2, and PD maps of an axial slice synthesized from a synthetic volume, together with the corresponding ground-truth maps. Further, it shows the pixelwise normalized-square-error (NSE) map between the synthesized and ground-truth maps. Similarly, Figure 3 shows the synthesized, ground-truth, and NSE maps from a representative slice of the real test volume. Finally, the evaluation metrics are shown in Table 1.Discussion
These preliminary results show the potential of the proposed approach to synthesize parametric maps. The synthesized maps from T1- and T2-weighted images for the synthetic dataset achieve NSE values below 1%. On the real test volume, the synthesized maps capture most of the structural information with NSE below 15%. Further, the metrics obtained are comparable with those obtained by state-of-the-art synthetic MRI methods2,3 to generate a synthetic image from one or more inputs. Note that the three synthesized parametric maps are obtained from only two weighted images, thus a fair comparison with traditional estimation techniques is not straightforward. Nevertheless, this work has several limitations, further validation with more in-vivo acquisitions and extensions to other pulse sequences (i.e., MPRAGE or DWI) are still needed.Conclusion
We propose an approach for the generation of synthetic T1, T2 and PD parametric maps which only needs two input images and which provides realistic outputs when trained solely with synthetic data. Results suggest its utility for quantitative MRI in clinical viable times as well as its applications for the synthesis of any image modality.Acknowledgements
The authors acknowledge grant TEC2017-82408-R from the Ministerio de Ciencia e Innovación of Spain.References
1. Treceño-Fernández D, et al. A Web-based Educational Magnetic Resonance Simulator: Design, Implementation and Testing. J Med Syst. 2019;In Press.
2. Jog A, et al. Random forest regression for magnetic resonance image synthesis. Med Image Anal. 2017;35:475–488.
3. Chartsias A, et al. Multimodal MR synthesis via modality-invariant latent representation. IEEE Trans Med Imaging. 2018;37(3):803–814.
4. Dar SUH, et al. Image synthesis in multi-contrast MRI with conditional generative adversarial networks. IEEE Trans Med Imaging. 2019.
5. Tofts P. Quantitative MRI of the brain: measuring changes caused by disease. John Wiley & Sons; 2015.
6. Larsson HBW, at al. In vivo determination of T1 and T2 in the brain of patients with severe but stable multiple sclerosis. Mag Reson Med. 1988;7(1):43-55.
7. Deoni SCL, et al. High-resolution T1 and T2 mapping of the brain in a clinically acceptable time with DESPOT1 and DESPOT2. Mag Reson Med. 2005;53(1):237–241.
8. Schmitt P, et al. Inversion recovery TrueFISP: quantification of T1, T2, and spin density. Mag Reson Med. 2004;51(4):661-667.
9. Ramos-Llordén G, et al. NOVIFAST: A fast algorithm for accurate and precise VFA MRI T1 mapping. IEEE Trans Med Imaging. 2018;37(11):2414–2427.
10. Ma D, et al. Magnetic resonance fingerprinting. Nature. 2013;495(7440):187-192.
11. Kwan RS, et al. MRI simulation-based evaluation of image-processing and classification methods. IEEE Trans Med Imaging. 1999;18(11):1085-1097.
12. Xiao Y, et al. A dataset of multi-contrast population-averaged brain MRI atlases of a Parkinson’s disease cohort. Data in brief. 2017;12:370-379.
13. Marek K, et al. The parkinson progression marker initiative (PPMI). Prog Neurobiol. 2011;95(4), 629-635.
14. Jenkinson M, at al. FSL. NeuroImage. 2012;62(2):782-790.
15. Bernstein MA, et al. Handbook of MRI pulse sequences. Elsevier. 2004.
Figures