3797
Variable Spatial Resolution Dual-Venc 4D Flow MRI: Balancing Image Quality and Scan Time
Maria Aristova1, Jianing Pang2, Liliana Ma1, Michael Markl1, and Susanne Schnell1
1Radiology, Northwestern University, Chicago, IL, United States, 2Siemens Healthcare, Chicago, IL, United States
1Radiology, Northwestern University, Chicago, IL, United States, 2Siemens Healthcare, Chicago, IL, United States
Synopsis
Dual-venc 4D flow MRI has previously been reported to achieve wide velocity dynamic range while maintaining a high velocity-to-noise ratio, which is particularly relevant for neurovascular applications. To increase flexibility in spatiotemporal resolution, we present a prototype implementation of a interleaved 8-point dual-venc 4D Flow acquisition with independently prescribed (prospectively undersampled) spatial resolution of the high venc acquisition, i.e. Variable Spatial Resolution Dual Venc (VSRDV). This allowed anti-aliasing error rates less than 15% error for a high venc spatial resolution of 70-80%. This represents 10-15% reduction of scan time.
Introduction
Dual-venc 4D flow MRI [1] has previously been reported to achieve wide velocity dynamic range while maintaining a high velocity-to-noise ratio. However, spatiotemporal resolution may be compromised in order to acquire dual venc data within clinically feasible scan times. Given that the high-venc data are only used for velocity aliasing correction, we hypothesize that the spatial resolution of the high-venc acquisition could be reduced in order to shorten the scan time, while still providing adequate information to correct velocity aliasing in the high-resolution, low-venc data. This approach has been investigated using retrospective undersampling of sequentially acquired low- and high-venc radial PC-VIPR [2], and a 2D phase-contrast implementation with interleaved 8-point dual-venc encoding [3]. Here we present a prototype implementation of a interleaved 8-point dual-venc 4D Flow acquisition with independently prescribed (prospectively undersampled) spatial resolution of the high venc acquisition, i.e. Variable Spatial Resolution Dual Venc (VSRDV).Methods
VSRDV 4D Flow was implemented in an 8-point dual-venc 4D Flow sequence with Cartesian sampling and variable phase-encoding resolution of the high venc acquisition (Figure 1). Reconstruction of both high- and low-venc images, Maxwell term correction, and low-venc velocity aliasing correction were integrated into the online reconstruction pipeline. The sequence was tested at 1.5 T (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany) on two in-vitro setups: a simple tube phantom with constant flow (Figure 2) and a realistic neurovascular anatomical replica phantom with pulsatile flow (Figure 3). Phase-encoding resolution of the high-venc acquisition was varied from 100% (used as reference) to 50% of that of low-venc with all other parameters identical. Performance was evaluated by the correct identification of aliased voxels. A comparison of flow and velocity measurements across multiple planes in each imaging volume was also performed.Results
In the tube phantom antialiasing error rate and specificity were within 15% of reference for high venc resolution values of 70, 80, and 90%. However, sensitivity was below 85% for all VSRDV values tested. Bland-Altman analysis for flow showed bias within 15% for VSRDV resolution of 60% and above; however, peak velocity showed high bias for all VSRDV resolutions tested. In the anatomical phantom antialiasing error rate and specificity were within 15% of reference for all high venc resolution values tested; sensitivity was lowest for 80% sampled data. Bland-Altman analysis for flow and peak velocity showed bias substantially within 15% for all VSRDV values in all vessels examined, except flow bias in the BA at 50% VSRDV.Discussion
Flow and velocity measurements for the tube phantom were within 15% of the reference for high-venc spatial resolutions of 70-80% and above. Flow and velocity measurements for the anatomical phantom were within 15% of the reference for high-venc resolutions of 80% and above. One potential confounding factor was segmentation error, though segmentations were based on the 100% sampled dual-venc data set.Conclusion
We successfully implemented a variable spatial resolution dual venc sequence, which allowed anti-aliasing error rates less than 15% for a high venc spatial resolution of 70-80%. This represents 10-15% reduction of scan time. VSRDV provides an opportunity for decreasing scan time required for dual-venc 4D Flow MRI while allowing to maximize temporal resolution and maintaining data quality. This may contribute to increased clinical applicability. Future work includes integration with acceleration methods such as GRAPPA, optimization of dual-venc phase unwrapping schemes and post-processing methods for VSRDV, and investigation of in-vivo performance.Acknowledgements
NIH F30 HL140910 (Aristova) NIH T32 GM815229 (Northwestern) NHLBI F30HL137279 (Ma) NIH R01 HL117888 (Markl) NIH R21NS106696 (Schnell) AHA 16SDG30420005 (Schnell) Circle of Willis model loan from United Biologics; Northwestern Research Shop staff.References
1. Schnell et al. J Magn Reason Imaging 2017;46:102–114. DOI: 10.1002/jmri.25595 2. Nett et al. J Magn Reson Imaging. 2012 Jun; 35(6): 1462–1471. DOI: 10.1002/jmri.23588 3. Aristova et al, Dual-venc phase contrast MRI with increased flow encoding efficiency. In: proceedings of the 28th ISMRM. Montréal, QC, Canada. May 11-16 2019.Figures
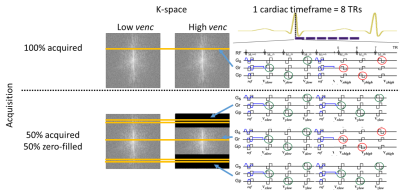
K-space acquisition scheme shown for a single
partition of VRSDV 4D Flow MRI. A cardiac time frame (8×TR) defines the temporal
resolution. (A) A single partition of 4D Flow MRI with full-resolution HV
requires e.g. 100 heartbeats to acquire 100 k-space lines. (B) 50% zero filling
with 2 LV k-space lines acquired per cardiac time frame at k-space periphery.
In an example case of 100 k-space lines the outer 50 LV k-space lines are
acquired in 25 cardiac time frames, which maintains the same temporal
resolution as (A) and reduces scan time to 75 heartbeats.
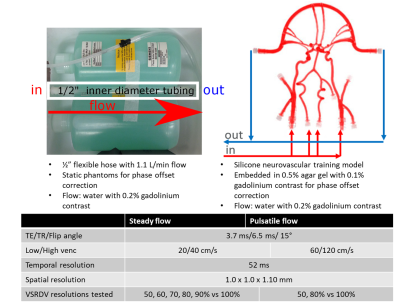
In vitro setup for (A) simple phantom with
steady flow and (B) neurovascular anatomical phantom with pulsatile flow. Scan
parameters for (C) simple phantom with steady flow and (D) neurovascular anatomical phantom with pulsatile flow.
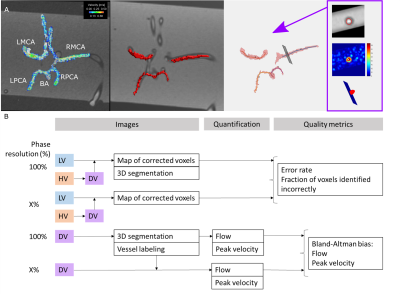
Post
processing workflow for (A) simple and (B) anatomical phantoms.
Data comparison schematic for (C) antialiasing performance and (D) hemodynamic
quantification performance in both phantoms.
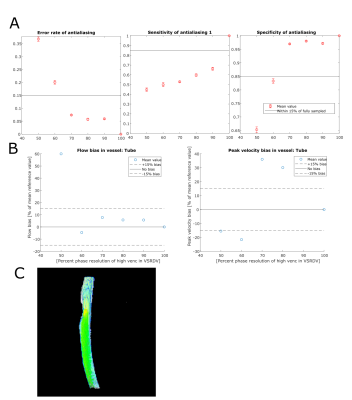
(A) Antialiasing performance and (B) hemodynamic
quantification performance in simple phantom with steady flow with (C) pathline
image at 80% phase sampling.
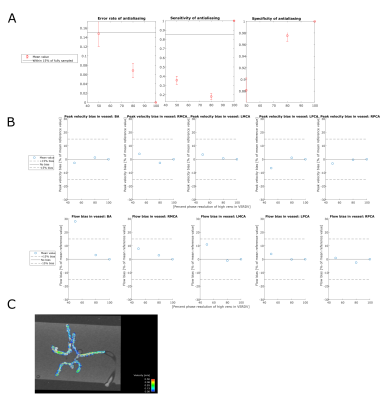
(A) Antialiasing performance and (B) hemodynamic
quantification performance in neurovascular anatomical phantom with pulsatile flow with (C)
pathline image at 80% phase sampling.