3782
Increasing robustness to fat partial volume effects in T1-mapping with MOLLI1ISR-Lisboa/LARSyS and Department of Bioengineering, Instituto Superior Técnico – Universidade de Lisboa, Lisbon, Portugal
Synopsis
Modified Look-Locker inversion recovery (MOLLI) with a balance Steady State Free Precession (bSSFP) readout is widely applied in the clinical setting but it is known to be sensitive to fat partial volume effects (PVE), as well as static B0 field inhomogeneities (ΔB0). As an alternative, the FLASH readout, more robust to ΔB0, can be applied for T1 mapping. In this work we studied the robustness of FLASH-MOLLI to fat PVE, and propose a new bi-component model to improve the accuracy of water T1 estimates in both bSSFP and FLASH MOLLI.
Introduction
T1 quantification enables the non-invasive characterization of tissue, providing relevant clinical information about the heart, the liver or even musculoskeletal regions.1-4 Inversion Recovery sequences with balance Steady State Free Precession (bSSFP) readouts are often used to measure the T1 parameter but this approach has been shown to be vulnerable to partial volume effects (PVE) which can arise from fat infiltration.1-3 In addition, the fat effect on T1 estimates may differ in the presence of static B0 field inhomogeneities (ΔB0).5 To improve the accuracy of the T1 estimates, this information can be separately measured and taken into account in the fitting. 1,6 An alternative method (FLASH), which relies on spoiled gradient echo readouts, is known to be more robust to ΔB0 and can also be applied to T1 mapping7, but the effect of fat in this context has not yet been studied. With this work, we evaluate the behaviour of T1 mapping with MOLLI using FLASH readout (FLASH-MOLLI) in the presence of fat. In addition, we propose a new bi-component model which accounts for the existence of fat avoiding T1 discrepancies due to PVE to both FLASH-MOLLI and bSSFP-MOLLI.Methods
MOLLI signals using acquisition schemes: 5(3)3, 3(3)3(3)5, and 4(3)3(3)2 for an RR=1000ms, with a bSSFP (FA=35º,TI1=100ms;TR/TE=2.5/1.25ms), and FLASH (FA=10º,TI1=120ms; TR/TE=3.6/1.4ms) readouts were simulated in Matlab2016a. Signals representing pure myocardial (T1/T2=1000/50ms; on-resonance) and fat tissues (T1/T2=260/60ms; 430Hz off-resonance)3 were combined to mimic fat fractions (FF) of [0.00-0.50]. Gaussian noise was added to obtain a typical SNR=80 at 3T. For both bSSFP-MOLLI and FLASH-MOLLI signals, T1 values were estimated with the conventional mono-component 3-parameter fitting 8 (Eq.1).$$S_{MOLLI}^{mono-component}\left(t,A,B,T_{1}^{*}\right)=A-B\exp\left\{-t/T_{1}^{*}\right\} \mathbf{Eq}(1)$$
The proposed bi-component model which accounts for both fat and water within the pixel (Eq.2) was also applied. In this model if a pixel is composed of two species, the signal can be expressed as:
$$\text{Signal}=\sum_{i=1}^{2}A_{i}-B_{i}\exp\left\{-\frac{t}{T_{1i}^{*}}\right\}\mathbf{Eq}(2)$$
$$\begin{array}{c}{\text{where}\quad A_{i}=M_{0i}^{*}=M_{0i}\frac{T_{1i}^{*}}{T_{1i}}}\\{\text{and} \quad B_{i}=M_{0 i}+M_{0i}^{*}=M_{0 i}\left(1+\frac{T_{1i}^{*}}{T_{1i}}\right)}\end{array}$$
since $$$\mathrm{M}_{0i}=\mathrm{F}_{i}\mathrm{M}_{0}$$$, ($$$F_i$$$ is the proton density fraction of component $$$i$$$), we can write:
$$\begin{array}{l}{\text{Signal}=\sum_{i=1}^{2}M_{0i}\frac{T_{1i}^{*}}{T_{1i}}-M_{0i}\left(1+\frac{T_{1i}^{*}}{T_{1i}}\right)\exp\left\{-\frac{t}{T_{1i}^{*}}\right\}} \\{=M_{0}\sum_{i=1}^{2}F_{i}\left[\frac{T_{1i}^{*}}{T_{1i}}-\left(1+\frac{T_{1i}^{*}}{T_{1i}}\right) \exp\left\{-\frac{t}{T_{1i}^{*}}\right\}\right]\quad}\end{array}\mathbf{Eq}(3)$$
Considering $$$P_i=T_{1i}^*/T_{1i}$$$:
$$\text{Signal}=M_{0}\sum_{i=1}^{2}F_{i}\left[P_{i}-\left(1+P_{i}\right)\exp\left\{-\frac{t}{P_{i} T_{1i}}\right\}\right]\mathbf{Eq}(4)$$
As the apparent T1 ($$$T_{1i}^*$$$) results from the readout effect, the following constraint is applicable: $$$0<P_i<1$$$.If $$$i=1$$$ and $$$i=2$$$ correspond to water ($$$w$$$) and fat ($$$f$$$), respectively, then $$$F_{i=1}=(1-FF)$$$, and $$$F_{i=2}=FF$$$, from which we obtain the bi-component model:
$$S_{MOLLI}^{bi-component}\left(t,P_w,P_f,T_{1w}\right)=M_{0}\left[(1-FF)\left(P_{w}-\left(P_{w}+1\right)\exp\left\{-\frac{t}{P_{w}T_{1w}}\right\}\right)+FF\left(P_{f}-\left(P_{f}+1\right)\exp\left\{-\frac{t}{P_{f} T_{1f}}\right\}\right)\right]\mathbf{Eq}(5)$$
By knowing the FF (e.g. estimated using the Dixon method), $$$M_0$$$ and fixing $$$T_{1f}$$$ to a value typical for T1 of fat, we can use Eq. 5 to estimate $$$P_w$$$, $$$P_f$$$, and $$$T_{1w}$$$. With this approach $$$T_{1w}$$$ is directly estimated, instead of having to apply a correction to an intermediate $$$T_{1w}^*$$$ value. This approach was applied to both FLASH-MOLLI and bSSFP-MOLLI signals.
Results
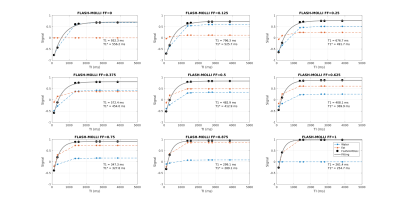
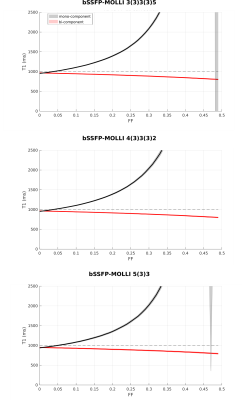
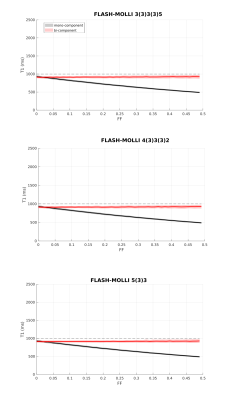
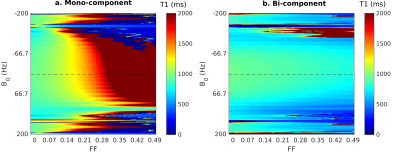
Figure 1 shows the signal evolution for FLASH-MOLLI at different fat fractions. The estimated T1w with mono-component 3-parameter fitting shows a similar behaviour to that presented by bSSFP-MOLLI when water and fat are in phase (i.e. T1w decrease with FF).3The new bi-component model was able to correct the bSSFP-MOLLI behaviour for the tested FF range, with bias/precision error of 100/20ms, while improving robustness to ΔB0 for higher FF values (Figure 4). However, the estimated T1w still shows a FF dependency (Figure 2). With the FLASH readout, the results from the new model become stable, presenting bias/precision errors of 67/20ms for the FF range tested (Figure 3).
Discussion
bSSFP is a widespread readout method applied in cardiac MRI due to its high SNR. Nevertheless, it is very sensitive to ΔB0, especially at high field strengths (i.e. 3T). This is particularly problematic in the presence of fat PVE. In contrast, the FLASH readout is known for its robustness to ΔB0, but that comes at a cost of low SNR. The ΔB0 robustness also explains why FLASH is less sensitive to fat infiltration than bSSFP, even with the standard mono-component 3-parameter fitting (Figure 1). Although bSSFP should enable a higher SNR which could improve precision, T1w values could be off by 50% for FF=0.20 when using the mono-component model, which could lead to an erroneous diagnosis.9The new bi-component model improved the accuracy of T1w estimates for both readouts, and presents the advantage of estimating T1w directly. The improvement for bSSFP-MOLLI shows that is possible to reduce bias for the sequences already available in clinical settings. Additionally, combining this method with FLASH-MOLLI has the potential to increase accuracy for FF by up to 50 %. Finally, this model would be readily applicable to new acquisition sequences recently proposed for T1 estimation which already include Dixon measurements.10
Conclusion
The bSSFP-MOLLI sequence is widely available in the clinic but it is known to be very sensitive to fat signal contamination. To account for its impact, clinically available methods such as Dixon measurements could be used for separately measure FF and this information used to estimate accurate T1W values with the proposed post-processing bi-component model fitting. This work shows that further improvements are also possible by using the new model in combination with a FLASH readout due to its robustness to ΔB0. Further investigations are underway to evaluate these results in vivo.Acknowledgements
Portuguese Foundation for Science and Technology (FCT - IF/00364/2013, UID/EEA/50009/2019, SFRH/BD/120006/2016), and POR Lisboa 2020 (LISBOA-01-0145-FEDER-029686).References
1. Mozes FE, Tunnicliffe EM, Moolla A, Marjot T, Levick CK, Pavlides M, et al. Mapping tissue water T1 in the liver using the MOLLI T1 method in the presence of fat, iron and B0 inhomogeneity. NMR Biomed. 32(2):e4030.
2. Larmour S, Chow K, Kellman P, Thompson RB. Characterization of T1 bias in skeletal muscle from fat in MOLLI and SASHA pulse sequences: Quantitative fat-fraction imaging with T1 mapping. Magn Reson Med. 2017;77(1):237–49.
3. Kellman P, Bandettini WP, Mancini C, Hammer-Hansen S, Hansen MS, Arai AE. Characterization of myocardial T1-mapping bias caused by intramyocardial fat in inversion recovery and saturation recovery techniques. J Cardiovasc Magn Reson. 2015;17(1):33.
4. Marty B, Carlier PG. Physiological and pathological skeletal muscle T1 changes quantified using a fast inversion-recovery radial NMR imaging sequence. Sci Rep. 2019;9(1):6852.
5. Mozes FE, Tunnicliffe EM, Pavlides M, Robson MD. Influence of fat on liver T1 measurements using modified Look-Locker inversion recovery (MOLLI) methods at 3T. J Magn Reson Imaging. 2016;44.
6. Liu D, Steingoetter A, Parker HL, Curcic J, Kozerke S. Accelerating MRI fat quantification using a signal model-based dictionary to assess gastric fat volume and distribution of fat fraction. Magn Reson Imaging. 2017;37:81–9.
7. Shao J, Rapacchi S, Nguyen K-L, Hu P. Myocardial T1 mapping at 3.0 tesla using an inversion recovery spoiled gradient echo readout and bloch equation simulation with slice profile correction (BLESSPC) T1 estimation algorithm. J Magn Reson Imaging. 2016;43(2):414–25.
8. Deichmann R, Haase A. Quantification of T1 values by SNAPSHOT-FLASH NMR imaging. J Magn Reson. 1992;96:608-612.
9. Bouazizi K, Rahhal A, Kusmia S, Evin M, Defrance C, Cluzel P, et al. Differentiation and quantification of fibrosis, fat and fatty fibrosis in human left atrial myocardium using ex vivo MRI. PLoS One. 2018;13(10):1–16.
10. Nezafat M, Nakamori S, Basha TA, Fahmy AS, Hauser T, Botnar RM. Imaging sequence for joint myocardial T1 mapping and fat/water separation. Magn Reson Med. 2019; 81: 486– 494.
Figures