3781
Simultaneous B1- and Fat-Corrected T1 Mapping Using Chemical-Shift Encoded MRI1Radiology, University of Wisconsin - Madison, Madison, WI, United States, 2Electrical and Computer Engineering, University of Wisconsin - Madison, Madison, WI, United States, 3Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 4Biomedical Engineering, University of Wisconsin - Madison, Madison, WI, United States, 5Medicine, University of Wisconsin - Madison, Madison, WI, United States, 6Emergency Medicine, University of Wisconsin - Madison, Madison, WI, United States
Synopsis
Spatially varying B1 inhomogeneities and tissue fat are known confounders of quantitative T1 mapping methods that use variable flip angle techniques. Separately acquired B1 calibration maps can be used to correct flip angle errors caused by B1 inhomogeneities, but this requires an additional acquisition. In this work we propose a novel approach for simultaneous estimation of B1, T1, proton density fat-fraction and R2* using dual orthogonal RF pulses and multiple flip angles. The feasibility and noise performance of this proposed acquisition and fitting strategy are evaluated using Cramer-Rao Lower Bound analysis, simulations, and preliminary phantom experiments.
Introduction
T1 is emerging as a quantitative biomarker of tissue fibrosis (the most important prognostic biomarker of nonalcoholic steatohepatitis) which can lead to cirrhosis and increase the risk of primary liver cancer1–3. T1 mapping using variable flip angle (VFA) is commonly used in the body where short acquisition times and motion robustness are important4,5. However, VFA is confounded by the presence of tissue fat and B1 inhomogeneities6. The effects of fat can be addressed through fat-water separated chemical shift encoded MRI (CSE-MRI) techniques7,8; however, standard B1-corrected VFA methods require a separate calibration acquisition to correct for B1 errors9.Therefore, the purpose of this work is to propose and present a novel B1- and fat-corrected CSE-MRI T1 mapping method. Our approach incorporates the orthogonal-$$$\alpha$$$ B1 mapping method10 with a traditional VFA strategy5 to jointly estimate B1- and fat-corrected T1, as well as proton density fat fraction (PDFF) and R2* maps without the need for an additional B1 calibration acquisition.
Theory
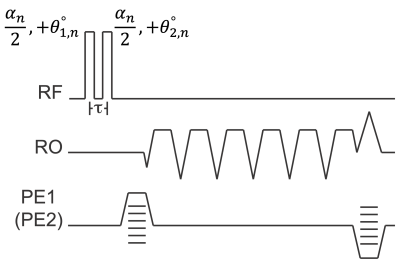
The orthogonal-$$$\alpha$$$ B1 mapping method10 uses two RF pulses of equal magnitude, but orthogonal in phase, in order to encode the actual flip angle into the phase of the free induction decay signal. This strategy exploits the non-commutative relationship of two rotations about orthogonal axes10. We propose a novel dual TR multi-echo VFA strategy that replaces each single RF excitation pulse with a composite excitation composed of two orthogonal RF pulses (Figure 1). Using the signal model of this acquisition strategy (derived using the MATLAB symbolic toolbox), non-linear least squares can be used to estimate β (B1 term, β relates the transmitted flip angle (αT) to prescribed flip angle (αP) by the equation αT=βαP), T1 of water and fat, PDFF, and R2*. Multi-peak modeling of fat is performed using a commonly used 6-peak spectral model11,12.Methods
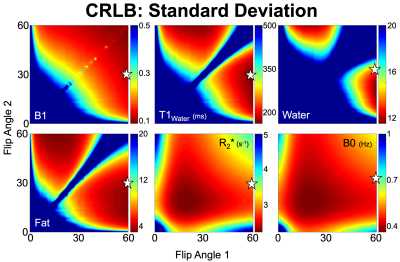
Optimization of Acquisition ParametersA Cramer-Rao Lower Bound (CRLB)13 was calculated for the acquisition strategy and the results were used to determine the set of acquisition parameters that would minimize the standard deviation of estimated T1W. Optimization was constrained to realizable TRs, TEs, and flip angles.
Numerical Simulations
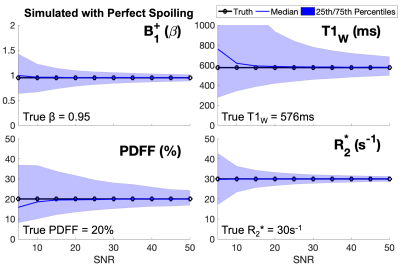
The proposed acquisition and nonlinear least squares joint reconstruction strategy were tested in simulated data using common 1.5T liver tissue parameters: β=0.95, T1W=576ms, T1F=280ms, PDFF=20%, R2*=3014,15 and acquisition parameters: α1=50o/α2=30o, TR1=11.6ms/TR2=11.6ms, TE1=1.08ms, ΔTE=1.7ms, echo train length (ETL)1=6, ETL2=6. For SNR ranging from 5-50 (SNR defined as: average |Sn|/σ, where σ=standard deviation of the noise), 10000 realizations of CSE-MRI were simulated with added zero-mean complex Gaussian noise. For each realization, a joint nonlinear least-squares fitting algorithm was used to estimate β, T1W, T1F, signal amplitude from water and fat and their shared initial phase, R2* and B0 fieldmap.
Phantom Acquisitions
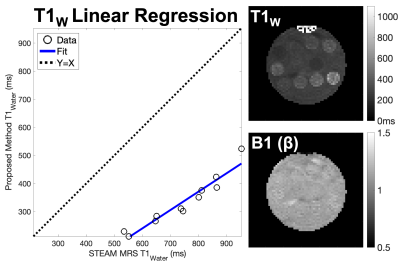
The proposed acquisition and reconstruction strategies were tested in a multi-vial agar gel phantom including varying T1W [approx. 400ms-800ms] and PDFF [approx. 0%-5%] values. Imaging was performed with a single channel quadrature head coil on a 1.5T system (GE Healthcare Optima MR450W, Waukesha, WI) using the same parameters listed in the numerical simulations and with the following acquisition parameters: 3D acquisition with 36 slices, 8mm slice thickness, 22x22cm2 FOV, ±62.50kHz bandwidth, 128x128 matrix and RF spoiling increment of 144.0o (selected to minimize phase errors). Multi-TE multi-TR spectroscopy (STEAM)16 was used as the reference for T1 and PDFF measurements. Parameters were estimated using nonlinear least squares and a region of interest (ROI) analysis compared our proposed method to STEAM-based T1W and PDFF in each vial.
Results
Optimization of Acquisition ParametersCRLB analysis determined optimal flip angles for T1W estimation to be 60o and 30o. Plots showing the CRLB optimization space are shown in Figure 2.
Numerical Simulations
Monte-Carlo simulations demonstrated the proposed strategy converges to unbiased estimators for all parameters of interest. Results of estimated β, T1W, R2*, and PDFF are shown in Figure 3.
Phantom Acquisitions
Results in phantom experiments showed good agreement with STEAM PDFF values. Estimates of T1W were highly correlated with STEAM T1W, but also exhibited bias. Results are shown in Figure 4.
Discussion
In this work we have described the successful development a novel B1- and fat-corrected CSE-MRI T1 mapping method. This strategy combines the orthogonal-$$$\alpha$$$ B1 mapping method with a traditional VFA strategy to estimate T1. Theoretical analysis was used to determine optimal acquisition parameters and performance was confirmed in numerical simulations.While phantom experiments demonstrated the proposed method’s ability to correct for both fat and B1, a substantial bias was observed in the estimates of T1W. We suspect this bias is partially explained by RF spoiling. While traditional RF spoiling ensures an accurate magnitude response, our method also requires an accurate phase response. Ongoing work will seek to correct the observed bias by implementing a look-up table reconstruction based on Bloch equation simulations, thus overcoming the undesirable tradeoffs of RF spoiling.
Experiments were limited to non-selective excitations; however, this method has important potential applications for rapid in vivo body T1 mapping. Future work will transition these preliminary concepts into a slab selective acquisition suitable for in vivo imaging.
Overall, the proposed strategy demonstrates initial feasibility for a rapid, breath hold eligible, B1- and fat-corrected T1-mapping technique without the need for a separate B1 calibration scan.
Acknowledgements
We wish to acknowledge support from the NIH (R01 DK088925, K24 DK102595, UL1TR002373), UW Institute for Clinical and Translational Research, and the Clinical and Translational Science Award of the NCATS/NIH. Further, we wish to acknowledge GE Healthcare who provides research support to the University of Wisconsin. Finally, Dr. Reeder is a Romnes Faculty Fellow, and has received an award provided by the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation.References
1. Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013;10:656–665 doi: 10.1038/nrgastro.2013.183.
2. Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999;30:1356–62 doi: 10.1002/hep.510300604.
3. Banerjee R, Pavlides M, Tunnicliffe EM, et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol 2014;60:69–77 doi: 10.1016/j.jhep.2013.09.002.
4. Brookes JA, Redpath TW, Gilbert FJ, Murray AD, Staff RT. Accuracy of T1 measurement in dynamic contrast-enhanced breast MRI using two- and three-dimensional variable flip angle fast low-angle shot. J. Magn. Reson. Imaging 1999;9:163–171 doi: 10.1002/(SICI)1522-2586(199902)9:2<163::AID-JMRI3>3.0.CO;2-L.
5. Deoni SC, Rutt BK, Peters TM. Rapid combined T1 and T2 mapping using gradient recalled acquisition in the steady state. Magn Reson Med 2003;49:515–26 doi: 10.1002/mrm.10407.
6. Deoni SC. Correction of main and transmit magnetic field (B0 and B1) inhomogeneity effects in multicomponent-driven equilibrium single-pulse observation of T1 and T2. Magn Reson Med 2011;65:1021–35 doi: 10.1002/mrm.22685.
7. Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med 2007;58:354–64 doi: 10.1002/mrm.21301.
8. Wang X, Hernando D, Wiens C, Reeder S. Fast T1 Correction for Fat Quantification Using a Dual-TR Chemical Shift Encoded MRI Acquisition. In: ; 2017.
9. Cheng H-LM, Wright GA. Rapid high-resolution T1 mapping by variable flip angles: Accurate and precise measurements in the presence of radiofrequency field inhomogeneity. Magn. Reson. Med. 2006;55:566–574 doi: 10.1002/mrm.20791.
10. Chang YV. Rapid B1 mapping using orthogonal, equal-amplitude radio-frequency pulses. Magn Reson Med 2012;67:718–723 doi: 10.1002/mrm.23051.
11. Hamilton G, Yokoo T, Bydder M, et al. In vivo characterization of the liver fat (1)H MR spectrum. NMR Biomed 2011;24:784–90 doi: 10.1002/nbm.1622.
12. Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med 2008;60:1122–34 doi: 10.1002/mrm.21737.
13. Scharf LL, Mcwhorter LT. Geometry of the Cramer-Rao Bound. Signal Process. 1993;31:301–311 doi: Doi 10.1016/0165-1684(93)90088-R.
14. Stanisz GJ, Odrobina EE, Pun J, et al. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn. Reson. Med. 2005;54:507–12 doi: 10.1002/mrm.20605.
15. Gold GE, Han E, Stainsby J, Wright G, Brittain J, Beaulieu C. Musculoskeletal MRI at 3.0 T: relaxation times and image contrast. AJR Am J Roentgenol 2004;183:343–51 doi: 10.2214/ajr.183.2.1830343.
Figures