3778
Cross vendor validation of Hybrid Multidimensional MRI in the non-invasive measurement of prostate tissue composition1Department of Radiology, University of Chicago, Chicago, IL, United States, 2Ingalls Memorial Hospital, Flossmoor, IL, United States
Synopsis
This study evaluates the consistency of HM-MRI for non-invasive measurement of prostate tissue composition on scanners from two MRI vendors. HM-MRI was performed on Philips Achieva 3T (with endorectal coil) and Siemens Skyra 3T (without endorectal coil) MRI scanners. HM-MRI metrics measured for cancerous and benign prostatic tissue using Philips and Siemens were similar, with slight variation due to different patients in each cohort. Diagnostic accuracy for detecting PCa using HM-MRI was similar for both MR vendors: Philips (AUC = 0.94-0.99, p<0.05) and Siemens (AUC = 0.83-0.98, p<0.05).
Introduction
MRI is increasingly being used for prostate cancer (PCa) diagnosis. However, there is large inter-reader variability in the interpretation of prostate multi-parametric MRI, and around 15-30% of clinically significant cancers are missed even by expert radiologists (1). A recent feasibility study showed that prostate tissue composition can be measured non-invasively using Hybrid Multidimensional MRI (HM-MRI) (2). HM-MRI exploits the distinct MR properties of prostate tissue components: stroma, epithelium and lumen (3) to measure tissue composition changes non-invasively using MRI and uses this as a biomarker for non-invasive PCa detection. This approach has the potential to improve PCa diagnosis and determine its aggressiveness. Another study validated prostate tissue composition measurement using HM-MRI with reference standard quantitative histology results from whole mount prostatectomy (4). These studies were done using a single MRI vendor (Philips) using endorectal coil at a single center. For HM-MRI to be truly acceptable as a screening protocol, feasibility studies using HM-MRI on MR scanners from multiple vendor, with and without endorectal coil need to performed. Therefore, this study evaluates the consistency of HM-MRI for non-invasive measurement of prostate tissue composition on scanners from two MRI vendors.Materials and Methods
In this prospective study, patients with known or suspected prostate cancer underwent MRI on a Philips Achieva 3T (n=20, mean age = 65 years) or Siemens Skyra 3T (n=9, mean age = 64 years) MRI scanner prior to undergoing subsequent biopsy. The HM-MRI sequence consisted of a spin-echo module with diffusion sensitizing gradients placed symmetrically about the 180⁰ pulse followed by single shot echo-planar imaging readout. HM-MRI scans included all combinations of TE = 47, 75, 100 ms and b-values = 0, 750, 1500 s/mm2 on the Philips scanner and TE = 63, 100, 140 ms and b-values = 0, 300, 800, 1400 s/mm2 on the Siemens scanner. Tissue composition (stroma, epithelium and lumen) in each voxel was calculated using a three-compartment signal model, with distinct, paired ADC and T2 values associated with each compartment, similar to the previous studies (2,4).$$ \frac{S}{S_0} =\sum_{n=1}^{n=3} V_n \times exp (-ADC_n \times b - \frac{TE}{T2_n}) $$
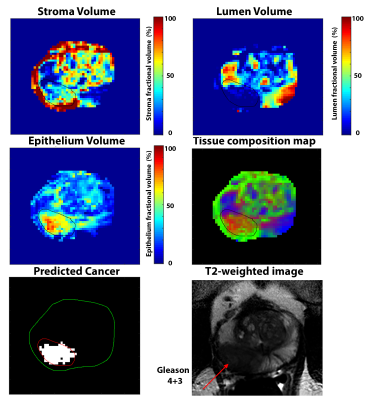
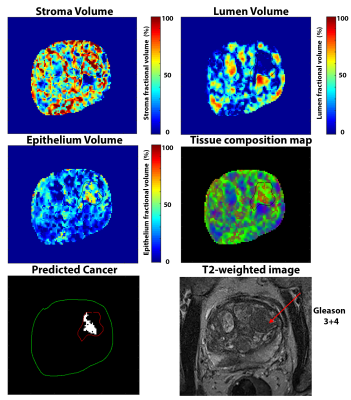
Suspected PCa with elevated epithelium (>40%) and reduced lumen (<20%) meeting the minimum size requirement of 25 mm2 on an axial slice were identified using the HM-MRI tool.
ADC maps was calculated using mono-exponential fit of HM-MRI signal at multiple b-values at TE = 75 ms on Philips and TE = 63 ms on Siemens cohort, while T2 maps were calculated from mono-exponential fit of HM-MRI signal at multiple TE values at b = 0 s/mm2 for both cohorts. ADC, T2 and tissue composition were calculated for ROIs drawn on biopsy-confirmed cancerous tissue and normal peripheral zone (PZ) and transition zone (TZ) tissue. HM-MRI metrics were compared for the two cohorts (different MR vendors) and with literature values. ROC analysis was performed to calculate area under the curve (AUC) to determine the diagnostic accuracy.
Results
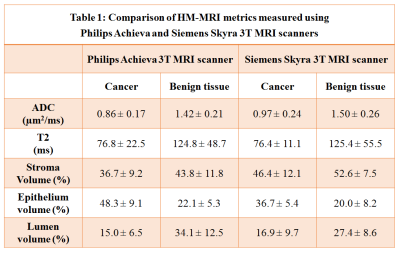
The Philips cohort involved 30 cancer ROIs (12 Gleason 3+3, 12 Gleason 3+4, 3 Gleason 4+3, 3 Gleason 4+5) and 40 benign PZ and TZ ROIs. The Siemens cohort involved 6 cancer ROIs (3 Gleason 3+3, 3 Gleason 3+4) and 16 benign PZ and TZ ROIs.HM-MRI metrics for benign prostatic tissue were significantly similar for Philips and Siemens cohorts (ADC: 1.42±0.21 vs 1.50±0.26 µm2/ms, p=0.33; T2: 124.8±48.7 vs 125.4±55.5 ms, p=0.97, Epithelium: 22.1±5.3 vs 20.0±8.2%, p=0.29, Lumen: 34.1± 12.5 vs 27.4±8.6%, p=0.09), except for stroma volume (43.8±11.8 vs 52.6±7.5%, p=0.02).
Similarly, metrics for cancer were also significantly similar for Philips and Siemens cohorts (ADC: 0.86±0.17 vs 0.97±0.24 µm2/ms, p=0.21; T2: 76.8±22.5 vs 76.4±11.1 ms, p=0.96, Lumen: 15.0±6.5 vs 16.9±9.7%, p=0.59), expect for stroma volume (Stroma: 36.7±9.2 vs 46.4±12.1%, p=0.04) and epithelium volume (48.3±9.1 vs 36.7±5.4%, p=0.01).
Cancer is characterized by significantly (p<0.05) increased epithelium and reduced lumen in both cohorts, but not stromal volume for one cohort (Siemens: p=0.21, Philips: p<0.05) The diagnostic accuracy for differentiating PCa from benign prostatic tissue based on the area under the curve (AUC) on ROC analysis were similar on Philips (AUC for epithelium = 0.99, lumen = 0.94; p<0.05) and Siemens (AUC for epithelium = 0.93, lumen = 0.83; p<0.05).
Discussion
Tissue composition HM-MRI was successfully implemented and validated to measure prostate tissue composition on two scanners from different vendors. HM-MRI tissue composition, ADC, and T2 were similar to literature values. Cancer is characterized by increased epithelium reduced lumen compared to surrounding benign prostatic tissue in both cohort, which is in agreement with histological studies (3,5). These results, consequently show that HM-MRI protocol with and without an endo-rectal coil is feasible.We expected some variation due to different patients in these cohorts and a cohort (Siemens) where lower Gleason grade cancers were imaged. This is evidenced by lower epithelium and higher stromal volume for cancers in the Siemens cohort compared to the Philips cohort. This variation in tissue composition is in agreement with a previous study on varying tissue composition of different Gleason patterns (6).
Conclusion
HM-MRI was successfully implemented and validated to measure prostate tissue composition on two scanners from different vendors. We are currently validating HM-MRI on GE scanners.Acknowledgements
No acknowledgement found.References
1. Niaf E, Lartizien C, Bratan F, Roche L, Rabilloud M, Mège-Lechevallier F, Rouvière O. Prostate Focal Peripheral Zone Lesions: Characterization at Multiparametric MR Imaging—Influence of a Computer-aided Diagnosis System. Radiology 2014;271(3):761-769.
2. Chatterjee A, Bourne R, Wang S, Devaraj A, Gallan AJ, Antic T, Karczmar GS, Oto A. Diagnosis of Prostate Cancer with Noninvasive Estimation of Prostate Tissue Composition by Using Hybrid Multidimensional MR Imaging: A Feasibility Study. Radiology 2018;287(3):864-872.
3. Bourne RM, Kurniawan N, Cowin G, Stait-Gardner T, Sved P, Watson G, Price WS. Microscopic diffusivity compartmentation in formalin-fixed prostate tissue. Magn Reson Med 2012;68(2):614-620.
4. Chatterjee A, Mercado C, Bourne RM, Yousuf A, Hess B, Antic T, Karczmar G, Oto A. Validation of prostate tissue composition measurement using Hybrid Multidimensional MRI: Correlation with quantitative histology. 2019; Montreal, Canada p0986.
5. Langer DL, van der Kwast TH, Evans AJ, Plotkin A, Trachtenberg J, Wilson BC, Haider MA. Prostate tissue composition and MR measurements: investigating the relationships between ADC, T2, K(trans), v(e), and corresponding histologic features. Radiology 2010;255(2):485-494.
6. Chatterjee A, Watson, G., Myint, E., Bourne, R.M. Changes in epithelium, stroma, and lumen space predict ADC changes with prostate cancer Gleason grade. In: Medicine ISfMRi, editor2014; Milan, Italy. p 4422.
Figures