3769
Quantitative and Synthetic MRI for Breast Assessment in Clinical Acquisition Times1Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 2High Field MR Centre, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 3Advanced Clinical Imaging Technology, Siemens Healthcare, Lausanne, Switzerland, 4Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 5LTS5, Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 6Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Contrast-enhanced breast MRI is the most sensitive tool for the detection of breast cancer. Contrast enhancement is however not specific to cancer: incidental benign findings regularly require further workup including ultimately avoidable follow-up scans and biopsies. Quantitative T1 and T2 measurements could be used as discriminative imaging markers to assist clinical decision-making. This feasibility study illustrates the potential clinical benefits of quantitative imaging for breast MRI by combining two fast and robust mapping techniques for high-resolution parameter mapping in a clinically feasible scan time of 7:32 min using prototype compressed sensing MP2RAGE and GRAPPATINI sequences with a harmonized protocol.
Introduction
Due to its superior sensitivity, breast MRI (bMRI) is an established diagnostic tool for screening in high-risk patients, management of unclear breast lesions and treatment planning in breast cancer patients [1,2]. However, the high sensitivity leads to the detection of benign enhancements, requiring further workup. Currently, qualitative image interpretation and diagnosis is done by combining semantic features such as enhancement patterns and kinetics based on relative T1/T2 signal intensities [3,4]. Qualitative interpretation, however, is both imperfect regarding its diagnostic information and experience-dependent – and might thus lead to potentially avoidable biopsies of benign lesions [5,6]. Biopsies of MRI-detected lesions are challenging: localization by ultrasound is frequently not possible and MRI-guided biopsies are both expensive and in short supply [7,8]. Therefore, objective and diagnostically helpful imaging markers are needed to further improve lesion characterization. Prior studies show promising results with quantitative T2 maps offering information about tumor biology and useful insights in monitoring treatment response [9,10]. We conducted a feasibility study employing two prototype sequences: GRAPPATINI[11,12] for accelerated T2 mapping and a compressed sensing MP2RAGE variant for T1 mapping for breast lesion characterization [13].Methods
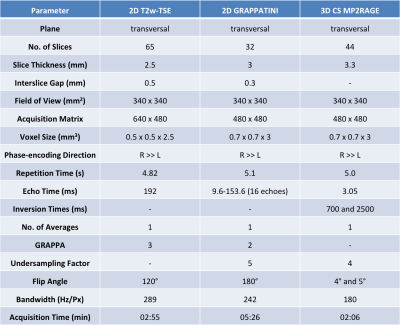
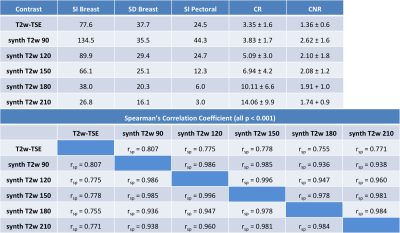
After written informed consent, 22 female patients (mean age 46.1 years, range 18 to 69 years) underwent bMRI at 3T (MAGNETOM Prismafit, Siemens Healthcare, Erlangen, Germany) using a 16-channel breast coil. GRAPPATINI was used with a five-fold undersampling resolved by the model-based reconstruction and additional two-fold GRAPPA, effectively resulting in ten-fold undersampled k-space data. Synthetic T2w contrasts were calculated at effective echo times (effTE) of 90, 120, 150, 180 and 210ms. Further, a prototype compressed-sensing-accelerated MP2RAGE sequence was performed with an acceleration factor of four [13] (Table 1). Final histology showed six malignant lesions, five after neoadjuvant chemotherapy, and two papillomas. A radiologist with two years of experience in bMRI assessed the international guideline compliant protocol [1]. Region of interests (ROI) were put manually on the T2w-TSE and copied to the T1 and T2 maps and synthetic T2w contrasts. Contrast ratios (CR) were calculated for the T2w-TSE as well as the synthetic T2 images at the different effTEs by dividing the signal intensity (SI) of the breast parenchyma by the SI of the pectoral muscle. Contrast-to-noise ratios (CNR) were calculated by subtracting the pectoral SI from the parenchyma SI and dividing the result by the standard deviation of the parenchyma ROI (Table 2).Results
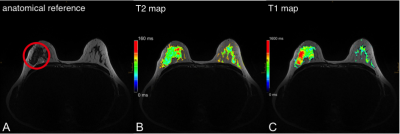
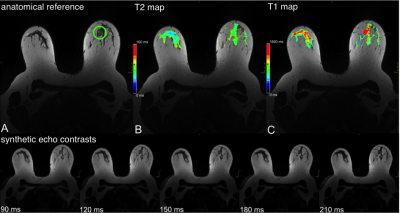
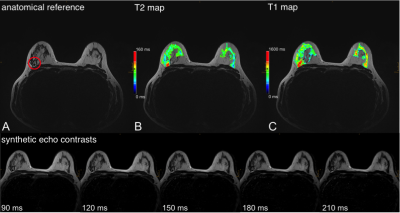
The resulting CRs in the synthetic contrasts were higher, especially with increasing effTE. The CR of the T2w-TSE was 3.4 ± 1.5 at 192ms TE compared to 10.1 ± 6.6 at effTE 190ms and 14.1 ± 9.9 at effTE 210ms (Table 2). The median T1 of healthy parenchyma was 1336.0 ± 422.2 ms and the median T2 was 77.6 ± 36.8 ms. Figure 1 shows a color-coded overlay of a 47-year-old patient with therapy-naïve right-sided breast cancer and lymphangiosis. Average T1 was 1528 ± 174ms in the lesion and 1104 ± 146ms in the healthy tissue while T2 was 91 ± 14ms and 69 ± 13ms. Figure 2 illustrates a left-sided retromamillary papilloma of an 18-year-old patient. T1 was 1738 ± 364ms in the lesion and 1445 ± 199ms in healthy tissue while T2 was 98 ± 88ms. and 71 ± 17ms respectively. Figure 3 shows a 35-year-old patient with left-sided breast cancer after neoadjuvant chemotherapy with good response and almost only necrotic tissue left. T1 was 1413 ± 230ms in the lesion and 1233 ± 151ms in the healthy tissue while T2 was 122 ± 20ms and 60 ± 13ms.Discussion
This proof-of-concept study illustrates the first application of a fast combination of a compressed sensing MP2RAGE prototype sequence (2:06 min) and GRAPPATINI (5:26 min) for bMRI. The illustrated cases in Figure 1 to 3 show aberrant T1 and T2 values for both malignant and benign lesions, with a clear trend for higher standard deviations in benign lesions. The CNRs of the synthetic morphological images demonstrate the potential application for better differentiation of signal loss behaviour in breast parenchyma and suspected lesions. Of note, the CNR in synthetic contrast may be artificially increased due to regularization in the image reconstruction. However, the CR – a noise-independent measure – showed the clear increase in differentiation of breast parenchyma to the pectoral muscle. This can be tweaked and exploited in clinical routine to increase discriminability. However, the different flip angle of the T2w-TSE and the slight offset in parameters limits this preliminary comparison. The diagnostic value of T2 mapping for lesion characterization [14] and monitoring of neoadjuvant chemotherapy [15] has been demonstrated. GRAPPATINI can additionally provide synthetic T2w contrasts alongside accelerated and robust T2 mapping. Reliable T1 mapping can be used for pharmacokinetic modelling of the signal intensity changes during dynamic contrast-enhanced sequences into actual contrast agent concentrations [16]. The presented compressed sensing MP2RAGE prototype provides fast T1 mapping while keeping the advantage of being a self-bias-field corrected sequence.Conclusions
We conclude that both T1w and T2w morphological contrasts and T1 and T2 maps can be derived using GRAPPATINI and the compressed sensing MP2RAGE prototype sequences with the potential to increase discriminability of malignant and benign lesions in bMRI.Acknowledgements
No acknowledgement found.References
1. Sardanelli F, Boetes C, Borisch B, et al. Magnetic resonance imaging of the breast: Recommendations from the EUSOMA working group. Eur J Cancer. 2010;46:1296–316.
2. Mann RM, Balleyguier C, Baltzer PA, et al. Breast MRI: EUSOBI recommendations for women’s information. Eur Radiol. 2015;25:3669–78.
3. American College of Radiology. ACR BI-RADS® ATLAS — BREAST MRI. 2013.
4. Dietzel M, Baltzer PAT. How to use the Kaiser score as a clinical decision rule for diagnosis in multiparametric breast MRI: a pictorial essay. Insights Imaging. 2018;9:325–35.
5. Baltzer PAT, Benndorf M, Dietzel M, et al. False-positive findings at contrast-enhanced breast MRI: a BI-RADS descriptor study. AJR Am J Roentgenol. 2010;194:1658–63.
6. Baltzer PAT, Kaiser WA, Dietzel M. Lesion type and reader experience affect the diagnostic accuracy of breast MRI: A multiple reader ROC study. Eur J Radiol. 2015;84:86–91.
7. Spick C, Baltzer PAT. Diagnostic utility of second-look US for breast lesions identified at MR imaging: systematic review and meta-analysis. Radiology. 2014;273:401–9.
8. Clauser P, Mann R, Athanasiou A, et al. A survey by the European Society of Breast Imaging on the utilisation of breast MRI in clinical practice. Eur Radiol. 2018;28:1909–18.
9. Bae MS, Shin SU, Ryu HS, et al. Pretreatment MR Imaging Features of Triple-Negative Breast Cancer: Association with Response to Neoadjuvant Chemotherapy and Recurrence-Free Survival. Radiology. 2016;281:392–400.
10. Uematsu T, Kasami M, Yuen S. Triple-Negative Breast Cancer: Correlation between MR Imaging and Pathologic Findings. Radiology. 2009;250:638–47.
11. Hilbert T, Sumpf TJ, Weiland E, et al. Accelerated T2 mapping combining parallel MRI and model-based reconstruction: GRAPPATINI. J Magn Reson Imaging JMRI. 2018;48:359–68.
12. Roux M, Hilbert T, Hussami M, et al. MRI T2 Mapping of the Knee Providing Synthetic Morphologic Images: Comparison to Conventional Turbo Spin-Echo MRI. Radiology. 2019;182843.
13. Mussard E, Hilbert T, Meuli R, et al. Accelerated MP 2 RAGE Imaging Using Sparse Iterative Reconstruction. 2015.
14. Liu L, Yin B, Shek K, et al. Role of quantitative analysis of T2 relaxation time in differentiating benign from malignant breast lesions. J Int Med Res. 2018;46:1928–35.
15. Liu L, Yin B, Geng DY, et al. Changes of T2 Relaxation Time From Neoadjuvant Chemotherapy in Breast Cancer Lesions. Iran J Radiol. 2016;13(3):e24014.
16. Rahbar H, Partridge SC. Multiparametric Breast MRI of Breast Cancer. Magn Reson Imaging Clin N Am. 2016;24:223–38.
Figures