3767
Fast T1 mapping with inversion recovery followed by radial acquisition and dictionary-matching reconstruction1MR Collaborations, Siemens Healthcare Ltd., Shanghai, China
Synopsis
A dictionary-based reconstruction method was applied to estimate T1 mapping from a series of highly undersampled images acquired with an inversion recovery–prepared FISP sequence and a radial readout. Motion robustness improved using interleaved slice acquisition and data rejection in T1 estimation. The measurement time was 4 s/slice. Phantom and in vivo knee results show an up to 50% data rejection results in less than 10% of quantification accuracy loss, compared to the T1 map reconstructed with the full dataset.
Introduction
Patients with Parkinson’s disease are with involuntary movements and require fast and motion-robust imaging techniques. Fast T1 mapping with an inversion recovery (IR)–prepared spoiled gradient echo (SPGR) sequence using radial acquisitions has been reported by Wang et al. 1 k-space samples at different inversion times (TIs) were grouped and reconstructed using a model-based sparse method. However, the image is a mixed contrast of data from different TIs. Modified Look-Locker inversion recovery (MOLLI) 2 is a frequently used fast T1-mapping technique for myocardia that uses a balanced steady-state free-precession (bSSFP) sequence with a Cartesian readout. The image weighting was mainly dependent on the inversion time of the central k-space line. Thus, MOLLI and its variants were sensitive to motion.3 Meanwhile, bSSFP-based sequences suffer from banding artifact especially at high field. In this study, a dictionary-based reconstruction method was applied to an IR-prepared FISP sequence with radial readouts to achieve fast and motion-robust T1 mapping.Methods
To maximize the signal-to-noise ratio (SNR), the excitation flip angle (FA) was optimized via Bloch simulation using different T1 and T2 relaxation times, and the results are shown in Figure 1. The optimal FA increased with T1 and T2 value increases. In this work, an FA of 15º and 20º was used for an in vivo knee study and a phantom study, respectively.Figure 2 shows the sequence diagram and reconstruction scheme. To improve motion robustness, radial readouts were used and associated with interleaved slice acquisitions after inversion preparation pulse. Radial spokes were rotated at a small golden angle of 23.6º to maximize the data incoherence between adjacent spokes from the same slice. With interleaved slice acquisition, the T1 sample redundancy allowed for a retrospective motion correction via data rejection. Each spoke was reconstructed to an image with high-undersampling aliasing using nonuniform Fast Fourier Transform (NUFFT)4, shown at the bottom of Figure 2.
A dictionary-based method was used to find the best match T1 value for each pixel, without changing the image contrast by directly combining the k-space data. The dictionary was generated using the IR signal model for all possible T1 values from the Bloch simulation.
Experiments were performed on a 3T MAGNETOM Prisma (Siemens Healthcare, Erlangen, Germany) using a 20-channel head coil and a 15-channel knee coil. The imaging parameters were an in-plane resolution = 1.0 × 1.0 mm2, slice thickness = 5 mm, repetition time (TR) = 8 ms, and echo time (TE) = 3.7 ms. The time interval between the inversion pulses was 8 s. Alternatively, to minimize the waiting time for volume recovery, only two slices were acquired, leaving other slices recovering. The acquisition time for each slice was 4 s.
Results
Figure 3 shows a slice from the phantom results. The image rejection rate increased from 10% to 50% for the pattern shown in the left column. Compared with the T1 map reconstructed with the full dataset, tubes with longer T1 maps were more robust at data dropping.Figure 4 shows the multi-slice knee results from the in vivo study. Images from slice numbers 1, 10, and 17 were selected and reconstructed using 30% and 50% data rejection. Compared with the T1 map reconstructed from the full dataset, the quantification error from the chosen region of interest (ROI; marked by a red box) was 6.8% and 9.3% for 30% and 50% data rejection, respectively.
Discussion
In this study, a fast and motion-robust T1 mapping method was implemented with IR-prepared radial acquisitions and a dictionary-based reconstruction. The proposed method was based on a FISP sequence, which should not have banding artifacts at high field strengths as seen with the bSSFP sequence used in MOLLI. Thus, the proposed method could achieve full FOV coverage, and could be applied to abdominal areas. Moreover, the proposed method had a higher SNR than the IR SPGR sequence, because the transverse magnetization was fully spoiled in the SPGR sequence.Dictionary-based reconstruction also improved motion tolerance in the proposed method. Motion influences for continuous TI samples on the same slice was reduced with an interleaved slice acquisition strategy. Thus, motion artifacts would not have a continuous impact on the TI samples from the same slice. The dictionary-based T1 estimation used temporal sparsity of signal changes to allow data dropping. The highly undersampled images from different TIs were not directly combined using any sparse reconstruction method. Therefore, the image contrast from different TIs was retained. However, streaking artifacts caused by radial undersampling were observed in both the phantom and in vivo knee studies. One possible solution is to apply a key-hole view-sharing strategy.5
Conclusions
In this study, we proposed a fast T1 mapping method with a high data rejection tolerance. This method directly benefits the use of T1 mapping in patients with Parkinson’s disease.Acknowledgements
No acknowledgement found.References
1. Wang X, Roeloffs V, Klosowski J, et al. Model‐based T1 mapping with sparsity constraints using single‐shot inversion‐recovery radial FLASH. Magn Reson Med, 2018, 79(2): 730-740.
2. Messroghli D, Radjenovic A, Kozerke S, et al. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med, 2004, 52(1): 141-146.
3. von Knobelsdorff-Brenkenhoff, F., Prothmann, M., Dieringer, M.A. et al. Myocardial T1 and T2 mapping at 3 T: reference values, influencing factors and implications. J Cardiovasc Magn Reson, 2013, 13:53.
4. Fessler JA, Sutton BP. Nonuniform fast Fourier transforms using min-max interpolation. IEEE Trans. Signal Process. 2003;51:560-574.
5. Ehses, P., Seiberlich, N, Ma, D, et al. IR TrueFISP with a golden‐ratio‐based radial readout: Fast quantification of T1, T2, and proton density. Magn Reson Med, 2013, 69: 71-81.
Figures
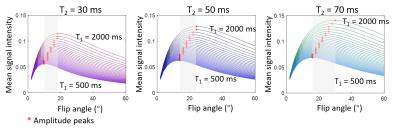
Figure 1. Simulations of the average signal intensity under different excitation flip angles using Bloch simulation. T1 varied from 500 ms to 2000 ms in a step of 50 ms. T2 increased from 30 ms in the left column, to 50 ms in the middle column, and to 70 ms in the right column.
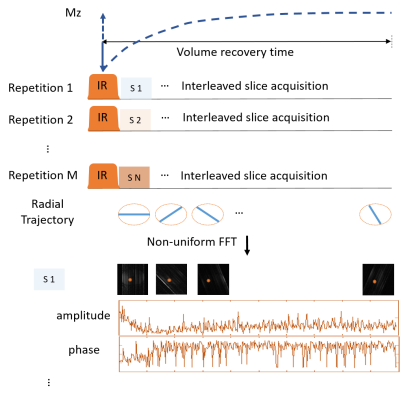
Figure 2. The sequence diagram and reconstruction method. k-space data were acquired with a slice-interleaved radial trajectory. The whole volume was inverted by an adiabatic inversion pulse.
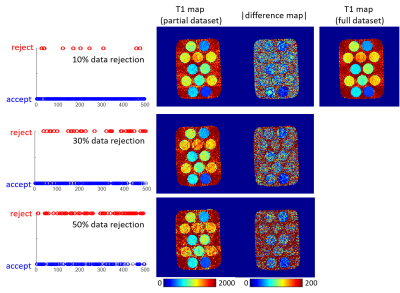
Figure 3. T1 maps of the phantom reconstructed using data rejection rates of 10%, 30%, and 50%. The tubes in the phantom were doped with different concentrations of MnCl2 to generate different T1 and T2 values. Data rejection patterns were generated by thresholding a random series shown on the left side.
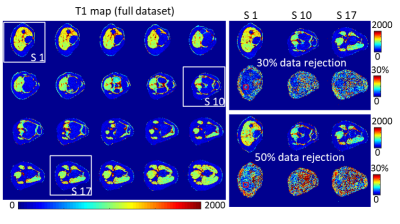
Figure 4. Multi-slice results of an in vivo knee. Slices (S1, S10, and S17) with different anatomy are selected and reconstructed using a data rejection of 30% and 50%. The error rates are shown at the bottom of reconstructed T1 maps.