3754
Simultaneous Multi-contrast Four-dimensional Magnetic Resonance Imaging for Radiotherapy Applications1The Hong Kong Polytechnic University, Hong Kong, Hong Kong, 2The University of Hong Kong, Hong Kong, Hong Kong
Synopsis
Tumor motion imaging is of vital importance in managing mobile cancers in radiation therapy. However, current 4D-MRI techniques are inefficient and ineffective, potentially leading to suboptimal and inaccurate results. To solve this problem, we propose a novel four-dimensional magnetic resonance fingerprinting (4D-MRF) technique for radiation therapy applications. Our proposed method has been validated through simulations and in-vivo volunteer experiment.
Introduction
Imaging has recently led to two paradigm shifts in radiotherapy in a hope to improve treatment efficacy, namely voxelization paradigm - the use of a nonuniform dose distribution that depends on the intratumural heterogeneity, and adaptation paradigm – detection and quantification of tissue changes during treatment with imaging.1 Altogether, the diagnostic value of and the accuracy in the estimation of tumor motion using imaging are pivotal to the reduction of uncertainties in and to the efficacy of radiotherapy. Magnetic resonance fingerprinting (MRF) is an emerging technique capable of simultaneously quantitative measurement of multiple tissue properties in a single scan. Its ability of reproducibility and quantitatively measurement is attractive to radiation therapy study but has not been explored. This study aims to investigate the feasibility of four-dimensional (4D) MRF technique for radiation therapy applications.Methods
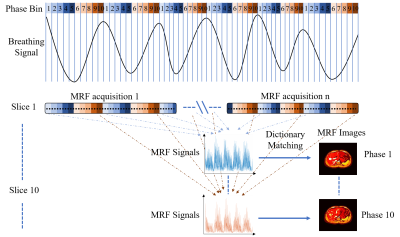
Abdominal T1, T2, and PD maps were generated using the extended cardiac-torso (XCAT) phantom for MRF simulation. The maximum diaphragm motion was 2 cm in cranial-caudal direction and 1.2 cm in anterior-posterior direction. The diameter of the tumor embedded in liver was 30 mm. Voxel size was 1.67 isotropic. The simulation of MRF data acquisition and reconstruction were performed in Matlab using in-house developed program. MRF acquisition with an inversion-recovery unbalanced steady-state free precession sequence2 was simulated using the extended phase graph algorithm. Regular and irregular breathing, during MRF acquisition were simulated. A variable density spiral-in-spiral-out readout trajectory with acquisition window = 8.4 ms and acceleration factor = 58.4 was used. The trajectory was rotated by a golden angle of 222.5o after each dynamic. The number of dynamics () = 1000, number of repetitions () = 10 (acquired after every 1000 dynamics), and 5 seconds were added between the end of one repetition and the beginning of the next to allow for signal recovery. Each MRF block of dynamics was triggered by different respiratory phases. Considering that the respiratory phases of the digital phantom is known, we can retrospectively identify the respiratory phase to which each MRF snapshot corresponds. Upon defining the number of bins in a respiratory cycle, the MRF snapshots that fall into a given respiratory bin can be determined. As a result, different groups of MRF snapshots will be used for the estimation of the MR parametric maps at different respiratory bins. The overall workflow of our proposed retrospective reconstruction is illustrated in Figure 1. The radiation therapy related quality index, including motion measurement accuracy, signal to noise ratio (SNR), contrast to noise ratio (CNR), and tumor volume consistency, of the corresponding reconstructed images were evaluated with XCAT phantom as gold standard. Three healthy volunteers were recruited to test the feasibility of our proposed method. MRI was performed using 3.0 Tesla human MRI scanner (Achieva TX, Philips Healthcare) with 8-channel head coil for signal reception.Results
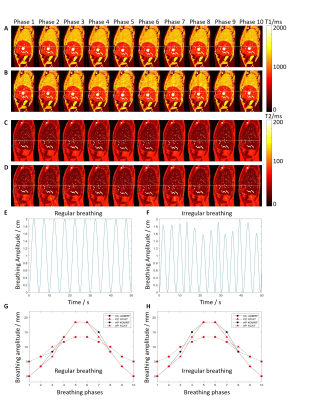
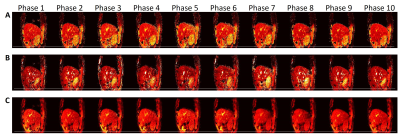
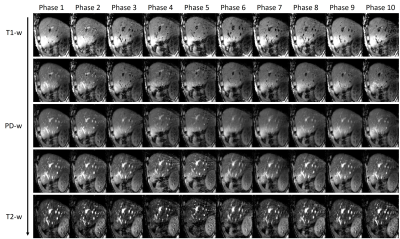
Dynamic MR parametric maps in the presence of regular and irregular breathing were successfully estimated from both XCAT phantom and healthy volunteer. The T1 and T2 maps of 5 respiratory phases in the presence of irregular (A and C) and regular (B and D) breathing are shown in Figure 2. The measured motion trajectories in CC and AP directions for both irregular (G) and regular breathing (H) are also shown in Figure 2. Numerical simulations showed that the TVE are 1.6 ± 2.7% and 1.3 ± 2.2% for irregular and regular breathing, respectively. The average absolute difference in tumor motion amplitude are 0.3 ± 0.7 mm and 0.3 ± 0.6 mm for irregular and regular breathing, respectively. The ADM were 4.1 ± 0.9% and 3.5 ± 0.9% for irregular and regular breathing, respectively. The SNR of the T1 and T2 maps of the liver and the tumor were generally higher for regular breathing than irregular breathing, whereas tumor-to-liver contrast is similar (within 0.1 difference) between the two breathing patterns. The proposed technique was also successfully implemented on the healthy volunteers. T1, T2, and PD maps of a representative volunteer are shown in Figure 3. T1-weighted, PD-weighted and T2-weighted images estimated from MR parametric maps are shown in Figure 4.Discussion
Current 4D-MRI techniques can only provide one type of weighted images for one scan, typically T1-weighted or T2-weighted3,4. However, different types of tumors may be better visualized using different weighted images. It is thus imperative that a better alternative to existing MRI methods for the estimation of motion be developed. Unlike conventional MRI, MRF allows for the simultaneous quantification of multiple tissue properties (T1, T2, proton-density, etc.) in a single, time-efficient acquisition. MRF has great potential to significantly improve the accuracy and work efficiency of treatment for abdominal cancers, as compared to CT and conventional MRI. We have successfully demonstrated that this gap may potentially be addressed using our proposed 4D-MRF. The respiratory motions and quantification of MR parameters at different respiratory phases can be reliably estimated using the recently proposed MRF technique5 in conjunction with our proposed retrospective reconstruction algorithm.Conclusion
We have successfully demonstrated the feasibility of a novel 4D-MRF technique that permits the estimation of quantitative parametric MR maps for different respiratory phases.Acknowledgements
No acknowledgement found.References
1. Jaffray DA. Image-guided radiotherapy: from current concept to future perspectives. Nat Rev Clin Oncol. 2012;9(12):688-699.
2. Jiang Y, Ma D, Seiberlich N, Gulani V, Griswold MA. MR fingerprinting using fast imaging with steady state precession (FISP) with spiral readout. Magn Reson Med. 2015;74(6):1621-1631.
3. Liu Y, Yin FF, Czito BG, Bashir MR, Cai J. T2-weighted four dimensional magnetic resonance imaging with result-driven phase sorting. Med Phys. 2015;42(8):4460-4471.
4. Cai J, Chang Z, Wang Z, Paul Segars W, Yin FF. Four-dimensional magnetic resonance imaging (4D-MRI) using image-based respiratory surrogate: a feasibility study. Med Phys. 2011;38(12):6384-6394.
5. Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature. 2013;495(7440):187-192.
Figures