3748
Reducing off-resonance effects by spatial decorrelation in Magnetic Resonance Fingerprinting1Biomedical Imaging Center, Pontificia Universidad Catolica de Chile, Santiago, Chile, 2Department of Electrical Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile, 3Millennium Nucleus for Cardiovascular Magnetic Resonance, Chile, Santiago, Chile, 4School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, London, United Kingdom, 5Institute for Biological and Medical Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile, Santiago, Chile
Synopsis
Magnetic Resonance Fingerprinting (MRF) acquisitions based on balanced Steady State Free Precession (bSSFP) with spiral trajectories produces higher signal noise to ratio (SNR) in comparison to the unbalanced version, nevertheless its usage is hider because of higher sensitivity to off-resonance artifacts. These artifacts affect the quality of parametric values. Here we propose a novel method to decrease these susceptibility distortions by means of spatial decorrelation. We show promising results where off-resonance artifacts were reduced in a realistic brain simulation and standardized T1/T2 phantom acquisition.
Introduction
Magnetic Resonance Fingerprinting (MRF) has been successful used to simultaneously estimate T1 and T2 values using a balanced Steady State Free Precession (bSSFP) acquisition1. It has been shown that MRF-bSSFP with spiral sampling can retain the spin history and achieves high signal to noise ratio (SNR) and scan efficiency2. However, banding artefacts produced by off-resonance effects in the bSSFP sequence can affect the quality of the parametric maps3. Moreover, the spiral acquisition causes blurring due to off-resonance3. Ostenson et al.4 proposed a correction for unbalanced SSFP, however this approach only considers smooth variations of field maps. A general theoretical approach to correct for off-resonance distortions has been proposed for conventional (non-MRF) spiral MR imaging5. Here we propose to extend this approach to correct off-resonance effects in MRF-bSSFP acquisitions. The feasibility of the proposed approach was evaluated in a realistic brain simulation and standardized T1/T2 phantom acquisition.Method
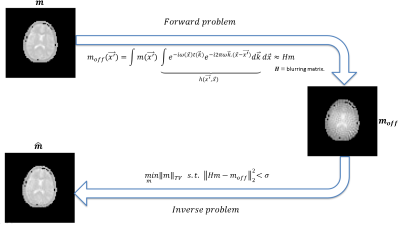
Off-resonance correction: to reduce the off-resonance artifacts we estimate the point spread function (PSF) for each TR simulating the sequence for a unitary pixel in all positions using the field map estimated by the conventional MRF. This allow us to build a blurring matrix H, such that:$$m_{off} = Hm \quad \quad (Eq.1)$$
where $$$m_{off}$$$ is the corrupted data and $$$m$$$ is the desired corrected data. For an $$$N\times N$$$ image, the $$$H$$$ matrix size is of $$$N^{2}\times N^{2}$$$ and the columns of $$$H$$$ corresponding to the pixel at location $$$(p,q)$$$ is obtained by column ordering of $$$PSF(p,q) = FFT^{-1} \{e^{-j\omega (p,q)t(r,s)}\} $$$ shifted by $$$(p,q)$$$, where $$$\omega$$$ is the off-resonance frequency and $$$t$$$ is the acquisition time. To estimate a corrected image, for each TR, we solved the inverse problem using Total Variation (TV) deblurring (see Fig. 1):
$$ \min_{m} ||m||_{TV} \quad s.t \quad ||Hm -m_{off}||_{2}^{2} \quad \quad (Eq.2) $$
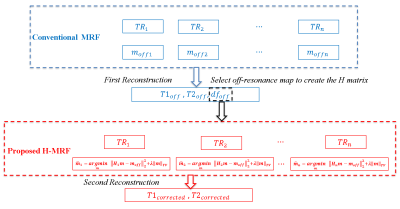
We used UNLocBoX7 (a convex optimization toolbox for MatLab) to solve the TV deblurring. Figure 2 shows a schematic summary of the comparison between the conventional MRF-bSSFP without off-resonance correction and the proposed matrix based on off-resonance correction approach (H-MRF).
Experiments: the proposed approach was tested in realistic brain simulations, and an acquisition on standardized T1/T2 phantom6 with two bottles of oil nearby to generate a distorted field map. The acquisition was performed on a 1.5 T scanner (Ingenia, Philips, Best, The Netherlands) using a 32‐element head coil. Relevant acquisition parameters included: Flip Angles (FA) between 0º-80º after an RF inversion pulse1, 1000 random Repetition Times (TR) between 14.5-18.0 ms, balanced gradients in each TR, and a spiral readout of 18 interleaved with 5.58 ms of readout time.The echo time (TE) was 7.25 ms, and was constant for each TR. The FOV was 128$$$\times$$$128 mm (simulations) and 288$$$\times$$$288 mm (phantom acquisitions) with in-plane resolution of 2$$$\times$$$2 mm and 8 mm slice thickness.
Dictionary generation: the fingerprints were generated using the Bloch equation for a range of T1, T2 and df (off-resonance) values: T1(400 to 2000 ms, steps of 10 ms), T2 (40 to 300 ms, steps of 4 ms), and df (off-resonance, -250 to 250 Hz, steps of 2 Hz). Parametric values were obtained by matching the measured signal to the closest dictionary entry using the maximum dot product.
Analysis of results: T1 and T2 values estimated with conventional MRF-bSSFP and the proposed H-MRF approach are compared using normalized root mean square error (NRMSE).
.
Results and discussion
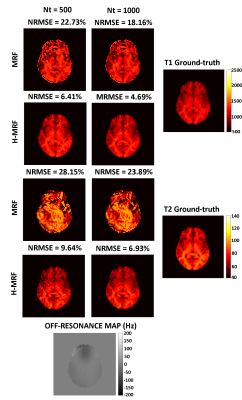
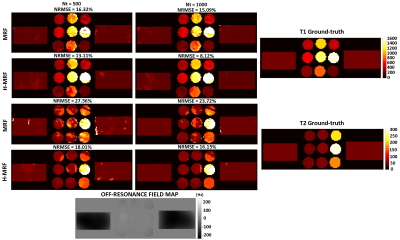
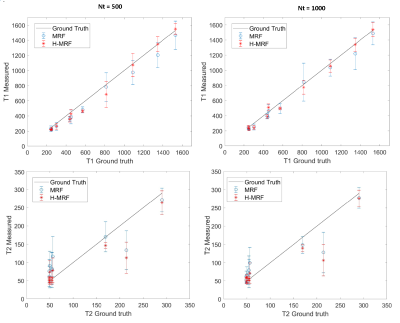
Reconstructed T1 and T2 maps using MRF and proposed H-MRF with different number of time points (Nt = 500 and Nt = 1000) are shown in Fig. 3 for the brain simulation. Strong artifacts are observed in both T1 and T2 maps with conventional MRF, whereas decreased artifacts are observed with the proposed H-MRF approach for both 500 and 1000 time points. NRMSE decreases for both methods with the number of time-points, however H-MRF achieved lower errors than conventional MRF. T2 NRMSEs at Nt = (500,1000) were (28.15%,23.89%) and (9.64%,6.93%) for MRF and H-MRF, respectively, whereas T1 NRMSEs at Nt = (500,1000) were (22.73%,18.16%) and (6.41%,4.69%) for MRF and H-MRF, respectively.Reconstructed T1 and T2 maps using MRF and proposed H-MRF with 500 and 1000 time points are shown in Fig. 4 for phantom acquisition. T1 and T2 values estimated with conventional MRF and H-MRF are compared against ground truth in Figure 5. Phantom acquisition presents similar results with both 500 and 1000 time points. H-MRF achieves lower T2 NRMSEs ((18.01%,16.15%) at Nt = (500,1000)) than conventional MRF ((27.56%,23.72%) at Nt = (500,1000)). Similarly, T1 NRMSEs are lower with H-MRF (13.11%,8.12%) in comparison to conventional MRF (16.32%,15.09%) at Nt = (500,1000). Figure 5 shows that H-MRF has better accuracy than conventional MRF for T2 values lower than 100 ms (brain tissues). H-MRF shows higher T1 precision in comparison to conventional MRF.
Conclusion
A novel and general approach H-MRF has been proposed to correct for off-resonance distortions in bSSFP-MRF with spiral sampling effects from corrupted images. H-MRF improved the quality of reconstructions in comparison to conventional MRF, particularly in its precision for brain tissues. Future work will be performed to validate the proposed approach in-vivo acquisitions.Acknowledgements
This publication has received funding from Millenium Science Initiative of the Ministry of Economy, Development and Tourism, grant Nucleus for Cardiovascular Magnetic Resonance.
Coronado R. thanks to CONICYT – PCHA/ Doctorado-Nacional/2018-21181628
References
1. Ma, D. et al., “Magnetic Resonance Fingerprinting”. Nature 495, 187–192 (2013).
2. Metha et al., “Magnetic resonance fingerprinting: a technical review”. Magnetic Resonance in Medicine (2018).
3. Nayak and Smith, “MRI artefacts and correction strategies”. Imaging Medicine, (2010).
4. Ostenson et al., “Multi-frequency interpolation in spiral magnetic resonance fingerprinting for correction of off-resonance blurring”. Magnetic Resonance Imaging, (2018).
5. Makhijani and Nayak, “Exact correction of sharply varying off-resonance effects in spiral MRI”. IEEE, (2006).
6. Captur et al. "A T1 and ECV phantom for global T1 mapping quality assurance: the T1 mapping and ECV standardisation in CMR (T1MES) program". J Cardiovasc Magn Reson. 2016;18:58.
7. Perraudin et al. "UNLocBoX: a MATLAB convex optimization toolbox for proximal-splitting methods". 2016. https://arxiv.org/pdf/1402.0779.pdf
Figures