3738
Motion Tracking using Continuous Magnetic Resonance Fingerprinting1Diagnostic Radiology, The University of Hong Kong, Hong Kong, Hong Kong, 2Health Technology and Informatics, The Hong Kong Polytechnic University, Hong Kong, Hong Kong, 3Philips Healthcare, Hong Kong, Hong Kong
Synopsis
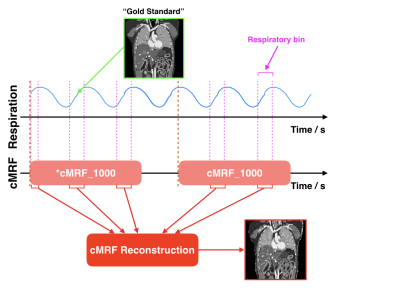
Unlike conventional contrast-weighted imaging, quantitative MR parametric maps can be obtained from MRF and should conceivably be very useful for the quantification and delineation of normal and pathologic tissues. Together with the fact that MRF is very efficient , our central hypothesis is that MRF is an ideal alternative to existing MRI motion tracking methods. In this study, we have demonstrated that it is possible to track motion by continuously performing MRF during free breathing. MR parametric maps for each respiratory phases were retrospectively estimated from the MRF snapshots that fall into a given respiratory bin.
Introduction
There are two broad categories of MRI methods for motion tracking: real-time acquisition 1 and retrospective reconstruction 2. The former has limited spatial resolutions whilst the latter can be confounded by irregular breathing 3. As retrospective reconstruction has significantly less requirements on scanner hardware and computing resources, it has been the method of choice 4. The keys to the reconstruction of time-resolved motion images using retrospective MRI methods relate to the temporal resolution of MRI acquisition and how the raw MRI data from different respiration phases and respiratory cycles are sorted and combined. Our current hypothesis is therefore that magnetic resonance fingerprinting (MRF) 5, a recently developed fast and quantitative MRI technique, could be a potential alternative to conventional motion tracking methods.Methods
Continuous MRFWe proposed a continuous MRF technique (cMRF) that permits subject to breath freely during the entire acquisition. cMRF is similar to conventional MRF but it is synchronized with respiratory and that there are more dynamic acquisitions to capture multiple respiratory cycles. We used the inversion-recovery unbalanced steady state free precession (IR-FISP) sequence and acquired 1000 dynamics in each spiral interleave with 10 repetitions that were preceded by 5-second delay. Images were acquired in the sagittal plane, where the out-of-plane motion is minimal 3. Respiratory traces were recorded using a pneumatic device strapped around the upper abdomen.
Experiments
Computer simulations were first performed to investigate the feasibility of cMRF for motion tracking. Physiological motions were simulated using the 4D Cardiac Torso digital phantom developed by Segars et al. 6. We have then performed in vivo experiments on a healthy volunteer. MRI experiments were performed using a 3.0 Tesla human MRI scanner (Achieva TX, Philips Healthcare) in a single slice. Other imaging parameters were: a constant-speed spiral-in-spiral-out readout trajectory (acquisition window of 8.4 ms, R = 58.4), trajectory rotation factor per dynamic = 222.5o, pseudorandomized TR (12 – 14.25 ms) and FA (0 – 60o), acquisition matrix = 256 x 256, image resolution = 1.17 x 1.17 mm2, slice thickness = 5 mm.
Retrospective MRF reconstruction
In the presence of motion, the dynamic images of neighboring TRs in a MRF dataset not only differ in signal intensity due to signal evolution but also spatial content. They are the “snapshots” of organs at different respiratory phases, and thus the conventional dictionary matching is no longer applicable. Nevertheless, we could retrospectively identify the respiratory phase to which each cMRF “snapshot” corresponded using the measured respiratory traces. Upon defining the number of bins in a respiratory cycle (i.e. respiratory bin), the cMRF snapshots that fall into a given respiratory bin could be determined (see the pink intervals in Figure 1). As a result, different groups of cMRF snapshots were used for the estimation of the MR parametric maps at different respiratory phases.
Results and Discussion
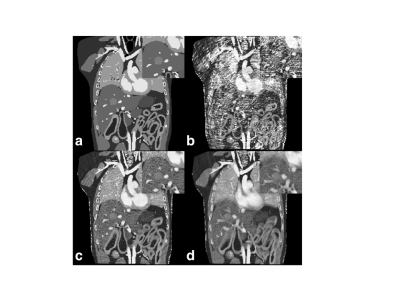
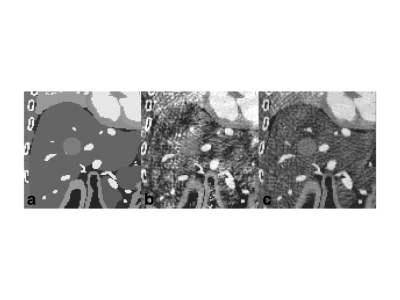
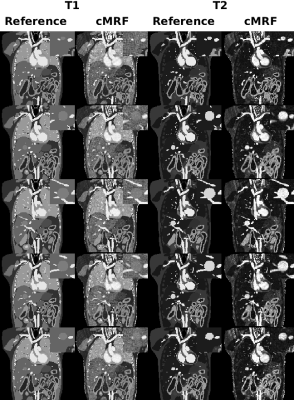
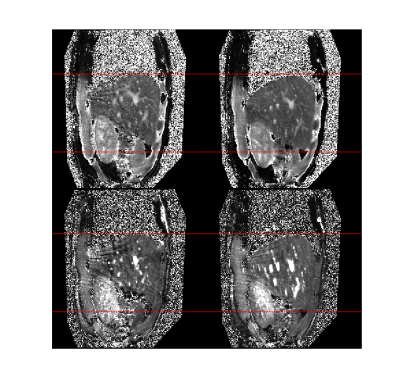
As demonstrated by numerical simulations (Figure 2), there is a trade-off between the respiratory bin resolution and the image quality of the MR parametric maps for different respiratory phases. As the resolution of the respiratory bin increases, the number of cMRF snapshots in the bin reduces, so does the fidelity of dictionary matching (Figure 2b). On the other hand, as the bin resolution decreases, the fidelity of dictionary matching is also compromised due to larger motion extent over more MRF dynamics. As is evident from Figure 2b, the image quality of the MR parametric maps obtained from cMRF warrants further improvement. An alternative strategy is to improve the quality of cMRF snapshots by using a sliding window reconstruction strategy 7. As shown in Figure 3b, the image quality of the MR parametric map is substantially improved for a sliding window size of twelve (i.e. each cMRF snapshot reconstructed using the k-space data from twelve consecutive dynamics).The MR parametric maps obtained from cMRF across different respiration phases largely correspond in spatial contents, such as image sharpness, size and shapes, to those from the 4D numerical phantom (see Figure 4). There is however slight image blurring in the intestines of the cMRF MR parametric maps (second and forth rows in Figure 4) and some artifacts throughout. Figure 5 shows the MR parametric maps of a healthy volunteer across two different respiratory phases.
Conclusion
We have successfully demonstrated that it is possible to track motion by continuously performing MRF during free breathing. In future studies, we aim to develop algorithm to directly estimate internal respiratory surrogate from cMRF data. This has the apparent advantage of obviating external pneumatic device.Acknowledgements
No acknowledgement found.References
1. Blackall, J. M. et al. MRI-based measurements of respiratory motion variability and assessment of imaging strategies for radiotherapy planning. Phys. Med. Biol. (2006). doi:10.1088/0031-9155/51/17/003
2. Von Siebenthal, M. et al. 4D MR imaging of respiratory organ motion and its variability. Phys. Med. Biol. (2007). doi:10.1088/0031-9155/52/6/001
3. Brandner, E. D., Chetty, I. J., Giaddui, T. G., Xiao, Y. & Huq, M. S. Motion management strategies and technical issues associated with stereotactic body radiotherapy of thoracic and upper abdominal tumors: A review from NRG oncology. Med. Phys. 44, 2595–2612 (2017).
4. Cai, J., Chang, Z., Wang, Z., Paul Segars, W. & Yin, F. F. Four-dimensional magnetic resonance imaging (4D-MRI) using image-based respiratory surrogate: A feasibility study. Med. Phys. (2011). doi:10.1118/1.3658737
5. Ma, D. et al. Magnetic resonance fingerprinting. Nature 495, 187–92 (2013).
6. Segars, W. P., Sturgeon, G., Mendonca, S., Grimes, J. & Tsui, B. M. W. 4D XCAT phantom for multimodality imaging research. Med. Phys. (2010). doi:10.1118/1.3480985
7. Cao, X. et al. Robust sliding-window reconstruction for Accelerating the acquisition of MR fingerprinting. Magn. Reson. Med. (2017). doi:10.1002/mrm.26521
Figures