3737
Generalised Low-Rank Non-rigid Motion Corrected reconstruction for 3D free breathing Liver Magnetic Resonance Fingerprinting1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Philips Healthcare, Guildford, United Kingdom
Synopsis
Magnetic Resonance Fingerprinting (MRF) has been shown to enable simultaneous T1 and T2 mapping of the liver and abdomen. 2D liver MRF requires breath holding, whereas preliminary results have been demonstrated for 3D free-breathing liver MRF using respiratory gating. However, gating approaches lead to unpredictable scan times and may impair the MRF encoding, since only data within the respiratory gating window is used for reconstruction. Here we propose a novel low-rank motion corrected approach to both resolve MRF varying contrast and perform non-rigid respiratory motion correction directly in the reconstruction, enabling 3D free-breathing liver MRF with 100% respiratory scan efficiency.
INTRODUCTION:
Magnetic Resonance Fingerprinting (MRF) enables simultaneous multi-parametric mapping1. Initially proposed for brain, MRF has also been extended for 2D liver and abdominal T1 and T2 mapping2, however with limited coverage. 3D free-breathing liver MRF preliminary results have been shown using respiratory gating (based on respiratory bellows)3. However, gating approaches lead to unpredictable scan times and may impair the MRF encoding, since only data within the end-expiratory respiratory gating window is used for reconstruction. A key challenge in 3D free-breathing abdominal MRF is the respiratory motion that can introduce artefacts in the parametric maps. Here we propose non-rigid respiratory motion compensated 3D free-breathing liver MRF with 100% scan efficiency. This is achieved with a novel LR-MC reconstruction that combines low rank6,7,8 and elastic motion corrected reconstruction9,10,11 to enable the contrast-resolved motion-corrected images required for MRF. The proposed 3D abdominal MRF approach was evaluated in 4 healthy subjects using an acquisition interrupted by variable preparation pulses, similar to previous 2D/3D abdominal2,3 and cardiac MRF4,5.METHODS:
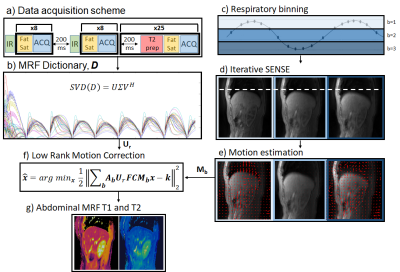
The proposed motion-corrected 3D abdominal MRF framework is outlined in Fig.1. Data is acquired free breathing with a stack of variable density spirals (Fig.1a). The “free-running” acquisition is interrupted by variable inversion recovery (IR) and T2 preparation (T2prep) pulses to induce T1 and T2 encoding, similar to previous 2D/3D abdominal2,3 and cardiac MRF4,5 approaches. In addition, spectral presaturation with IR (SPIR) pulses are used for fat suppression. The acquisition was segmented into shots with 30 spiral readouts per shot; with preparation pulses occurring between shots. The scheme in Fig.1a is repeated for each slice encoding. Low rank compression is derived via the MRF dictionary (Fig.1b). Low rank inversion (LRI)7 is used in MRF to compress the large number of acquired time-points to a few number of singular images (using singular value decomposition of the MRF dictionary). The proposed reconstruction consists of two steps: 1) non-rigid respiratory motion estimation and 2) LR-MC reconstruction. Motion estimation: the acquired data is binned into different respiratory bins using the 1D motion signal from respiratory bellows (Fig1.c). Auxiliary respiratory motion resolved images are reconstructed with iterative SENSE12 (Fig.1d) and registered13 to estimate respiratory motion (Fig.1e). LR-MC reconstruction (Fig.1f): The estimated motion fields for each respiratory bin b (step 1) are then incorporated into the proposed low rank motion corrected reconstruction, formulated as:$$\bf{x} = argmin_x \parallel \bf{\sum_b A_bU_rFCM_bx-k} \parallel _2^2$$
where, $$$U_r$$$, $$$F$$$ and $$$C$$$ are dictionary-based compression, non-uniform Fast Fourier Transform and coil sensitivity operators; $$$x$$$ are the singular images for the respiratory motion corrected MRF data, $$$k$$$ is the acquired k-space data, $$$A_b$$$ and $$$M_b$$$ are respectively the sampling matrices and the sparse motion matrices for the b-th motion state. T1 and T2 maps (Fig.1g) are obtained from the LR-MC reconstructed singular images via inner product with the compressed dictionary.
EXPERIMENTS:
Four healthy subjects were scanned under free-breathing at 1.5T (Philips Ingenia) with the proposed approach. Key parameters included: field of view (FOV) = 282x282x240 mm3; resolution = 1.6x1.6x8 mm3; 30 slices; TE/TR = 0.61/7.2; gradient echo; 6-10º sinusoidally varying flip angle; 3 second recovery between slice-encodings; sagittal orientation; acquisition time = 10 mins. Data was reconstructed with the proposed LR-MC reconstruction (considering 3 respiratory bins) for respiratory motion correction and with LRI reconstruction without motion correction.RESULTS:
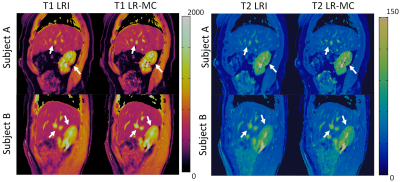
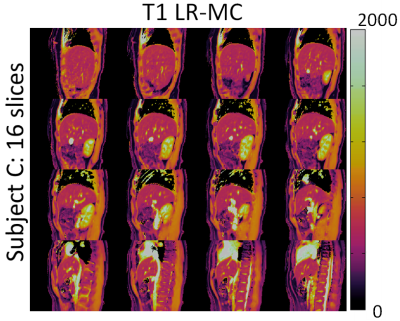
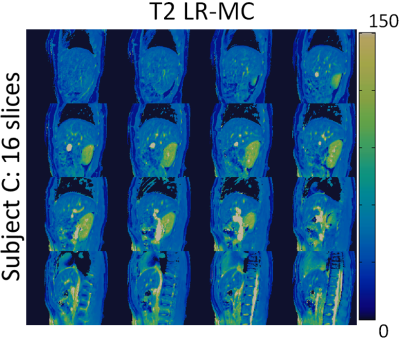
3D abdominal MRF without motion correction (LRI) produced considerable blurring artefacts in T1 and T2 maps, whereas these effects were reduced with the proposed LR-MC reconstruction as seen in Figure 2 for two representative subjects. T1 and T2 values in the liver, skeletal muscle and kidney measured with the proposed 3D abdominal MRF approach were in general agreement with literature values14,15 (Table 1), however an underestimation of T2 was observed, similar to previous reports in abdominal MRF2,3. Good delineation of the abdominal anatomy was observed for all slices across the whole-liver coverage, as seen in Figures 3 and 4 for T1 and T2 maps, respectively.CONCLUSION:
3D free-breathing abdominal MRF was proposed for simultaneous T1/T2 mapping, enabled by a novel low-rank 3D non-rigid motion corrected reconstruction. Future work will consider sequence optimizations towards improved scan efficiency and validation in a larger subject cohort. The proposed contrast resolved, motion corrected LR-MC reconstruction could be applied to other multi-contrast/multi-parametric applications and will be investigated as future work.Acknowledgements
ACKNOWLEGDMENTS: This work was supported by EPSRC (EP/P001009, EP/P032311/1) and Wellcome EPSRC Centre for Medical Engineering (NS/ A000049/1).References
1. Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, Griswold MA. Magnetic resonance fingerprinting. Nature 2013;495:187–192.
2. Chen Y, Jiang Y, Pahwa S, Ma D, Lu L, Twieg MD, Wright KL, Seiberlich N, Griswold MA, Gulani V. MR fingerprinting for rapid quantitative abdominal imaging. Radiology. 2016 Jan 21;279(1):278-86.
3. Ropella-Panagis K, Chen Y, Jiang Y, et al. Three-Dimensional, Free-Breathing Magnetic Resonance Fingerprinting for Whole-Liver Coverage. ISMRM 2019; abstract number 4378.
4. Hamilton JI, Jiang Y, Chen Y, et al. MR fingerprinting for rapid quantification of myocardial T 1 , T 2 , and proton spin density. MRM 2017;77:1446–1458.
5. Cruz G, Jaubert O, Schneider T, Bustin A, Botnar Rm, Prieto C. Toward 3D Free-breathing Cardiac Magnetic Resonance Fingerprinting. ISMRM 2019; abstract number 4385.
6. McGivney DF, Pierre E, Ma D, et al. SVD compression for magnetic resonance fingerprinting in the time domain. IEEE Trans. Med. Imaging 2014;33:2311–2322 doi: 10.1109/TMI.2014.2337321.
7. Zhao B, Setsompop K, Adalsteinsson E, et al. Improved magnetic resonance fingerprinting reconstruction with low-rank and subspace modeling. Magn. Reson. Med. 2018;79:933–942 doi: 10.1002/mrm.26701.
8. Assländer J, Cloos MA, Knoll F, Sodickson DK, Hennig J, Lattanzi R. Low rank alternating direction method of multipliers reconstruction for MR fingerprinting. Magn. Reson. Med. 2018;79:83–96 doi: 10.1002/mrm.26639.
9. Batchelor PG, Atkinson D, Irarrazaval P, Hill DLG, Hajnal J, Larkman D. Matrix description of general motion correction applied to multishot images. Magn. Reson. Med. 2005;54:1273–1280 doi: 10.1002/mrm.20656.
10. Odille F, Vuissoz PA, Marie PY, Felblinger J. Generalized reconstruction by inversion of coupled systems (GRICS) applied to free-breathing MRI. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2008 Jul;60(1):146-57.
11. Cruz G, Atkinson D, Buerger C, Schaeffter T, Prieto C. Accelerated motion corrected three-dimensional abdominal MRI using total variation regularized SENSE reconstruction. Magnetic resonance in medicine. 2016 Apr;75(4):1484-98.
12. Pruessmann KP, Weiger M, Börnert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2001 Oct;46(4):638-51.
13. Modat M, Ridgway G, Taylor Z, Lehmann M, Barnes J, Hawkes D, Fox N, Ourselin S. Fast free-form deformation using graphics processing units. Comput Meth Prog Bio 2010; 98: 278– 284.
14. Stanisz GJ, Odrobina EE, Pun J, Escaravage M, Graham SJ, Bronskill MJ, Henkelman RM. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2005 Sep;54(3):507-12.
15. Wolf M, de Boer A, Sharma K, Boor P, Leiner T, Sunder-Plassmann G, Moser E, Caroli A, Jerome NP. Magnetic resonance imaging T1-and T2-mapping to assess renal structure and function: a systematic review and statement paper. Nephrology Dialysis Transplantation. 2018 Aug 23;33(suppl_2):ii41-50.
Figures