3735
Single-shot Pseudo-Centric EPI for Magnetization-Prepared Imaging
Hyun-Soo Lee1, Seon-ha Hwang1, Jaeseok Park2, and Sung-Hong Park1
1Department of Bio and Brain Engineering, KAIST, Daejeon, Korea, Republic of, 2Department of Biomedical Engineering, Sungkyunkwan University, Suwon, Korea, Republic of
1Department of Bio and Brain Engineering, KAIST, Daejeon, Korea, Republic of, 2Department of Biomedical Engineering, Sungkyunkwan University, Suwon, Korea, Republic of
Synopsis
Single-shot EPI is a famous ultra-fast MR imaging technique, but is limited to linear reordering due to its special k-space trajectory. In this study, we proposed a single-shot pseudo-centric EPI where k-space is encoded from center to periphery in a groupwise manner by utilizing grouped oscillating readout gradients, phase‑encoding blips within each group, and big phase‑encoding jumps between two consecutive groups. The concept was tested on phantoms and human brains in 3T and 7T. The proposed method enabled the significant reduction of TE, which is expected to maximize SNR of magnetization-prepared imaging and enable ultrashort-TE imaging in the Cartesian coordinate.
Introduction
Single-shot EPI (ss-EPI) is the representative fast MR imaging technique where rapidly oscillating readout gradients and phase encoding (PE) blips are incorporated to acquire a full k-space after a single RF excitation1. Thus, ss-EPI is widely used as readout sequence for various MR imaging techniques such as diffusion, perfusion, and fMRI2-4. However, ssEPI can only be achieved with a linear PE order due to its innate k-space trajectory. For physiological imaging (i.e., diffusion, perfusion, magnetization transfer, inversion recovery imaging), which requires specific magnetization preparation before the data acquisition, a centric PE reordering where the center of the k-space is acquired first is preferred to the linear PE order in order to maximize SNR5,6. In the previous study, a phase encoding grouping (PE-grouping) was suggested for reducing eddy-current artifacts in centric-reordered balanced steady-state free precession (bSSFP)7. In this study, we utilized the PE-grouping in single-shot EPI to achieve single-shot pseudo-centric EPI. The proposed single-shot pseudo-centric EPI can significantly reduce the echo time (TE) to achieve better SNR for magnetization-prepared imaging. We also tested two phase correction methods to reduce the phase errors in the single-shot pseudo-centric EPI.Methods
(Pulse sequence) The single-shot pseudo-centric EPI was implemented as described in Fig.1. In single-shot pseudo-centric EPI, readout gradients with a specific number (N) were grouped and these groups are linearly encoded from the k-space center to the edge. Minimum-phase RF pulse was used in order to further reduce TE.(Phase correction) For conventional phase correction, navigators with three echoes without PE gradients were obtained before the data acquisition (i.e., three-echo phase correction method). For extensive phase correction for single-shot pseudo-centric EPI data, the whole k-space was acquired without PE gradients for every reordering method (i.e., whole-echo phase correction method) and the phase information was used for phase correction for each PE line.
(Data Acquisition) All experiments were performed on a 3T MRI (Skyra, Siemens, Erlangen) with a 16-ch head coil and a 7T MRI (Magnetom, Siemens, Erlangen) with a 32-ch head coil. The same parameters except the flip angle were used for the phantom and in vivo experiments and the parameters were as follows: TR = 300 ms, TE = minimum, matrix size= 128, FOV = 256192 , slice thickness = 5 mm , FA = 60(3T/7T, phantom)/35(3T, in vivo)/30(7T, in vivo). For linear ordering, partial Fourier of 3/4 was applied for reducing TE. Fat suppression was used for brain imaging.
Results
The minimum TEs were significantly reduced from 53.7 ms of linear ordering to 1.4 ms of centric reordering while the readout durations remained similarly with the difference < 6 ms for N>=4, which is acceptable in consideration of the total readout duration (Fig.2). In Fig.3, single-shot pseudo-centric EPI images (N=4) showed geometric distortions and signal dropout (indicated by yellow arrows) at the anterior part of the brain image with conventional three-echo phase correction method. However, most of the distortions were removed and blurring due to phase errors were profoundly reduced in the whole-echo phase correction method. In Fig.4-5, doped water phantom and representative human brain EPI images with proposed single-shot pseudo-centric EPI with various Ns (N=2,4, and 8) were shown. The images were proven to achieve comparable quality with 2-shot center-out EPI images at both 3 and 7T MRI.Discussion & Conclusion
In this study, we showed the possibility of centric reordering in single-shot EPI for the first time. With the proposed centric reordering, TE could be reduced to ~ 1 ms, which is expected to maximize SNR of magnetization‑ prepared physiological imaging and be applied to ultrashort TE imaging in the Cartesian coordinate for short T2* component imaging. With proposed whole-echo phase correction method, most of the distortions of the pseudo-centric EPI images were suppressed, thus choice of phase correction method was critical for the proposed reordering scheme. Although single-shot pseudo-centric EPI images were a bit blurry compared to 2-shot center-out EPI images, the image quality can be improved by averaging in case of magnetization-prepared imaging. Furthermore, for magnetization-prepared imaging, the proposed single-shot pseudo-centric EPI is advantageous in that it only requires single preparation time before compared to the doubled preparation time of the 2-shot center-out EPI. In conclusion, the proposed approach will improve the image quality of magnetization-prepared imaging by reducing TE significantly and enable UTE imaging in the Cartesian coordinate for faster image reconstruction.Acknowledgements
No acknowledgement found.References
1. Mansfield P. Multi-planar image formation using NMR spin echoes. J. Physics C 1977;10:L5-L582. Turner R, Le Bihan D, Maier J, Vavrek R, Hedges LK, Pekar J. Echo-planar imaging of intravoxel incoherent motion. Radiology 1990;177:407–414.
3. Poncelet BP, Koelling TM, Schmidt CJ, Kwong KK, Reese TG, Ledden P, Kantor HL, Brady T. Measurement of human myocardial perfusion by double‐gated flow alternating inversion recovery EPI. Magn Reson Med 1999; 41: 510– 519.
4. Kwong KK. Functional magnetic resonance imaging with echo planar imaging. Magn Reson 1995; Q 11:1–20.
5. Spincemaille P, Nguyen TD, Wang Y. View ordering for magnetization prepared steady state free precession acquisition: application in contrast-enhanced MR angiography. Magn Reson Med 2004;52:461–466.
6. Holsinger AE, Riederer SJ. The importance of phase-encoding order in ultra-short TR snapshot MR imaging. Magn Reson Med 1990;16:481–488.
7. Lee H-S, Choi S, Park S-H. Single and double acquisition strategies for compensation of artifacts from eddy current and transient oscillation in balanced steady-state free precession. Magn Reson Med 2017; 78(1): 254-263
Figures
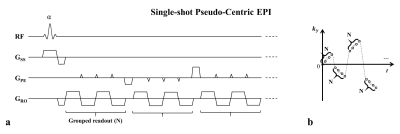
Fig. 1. Schematic
illustration of a single-shot pseudo-centric EPI. (a) Pulse sequence diagram. (b) K-space trajectory. After a single RF excitation, a certain
number of groups of oscillating readout gradients and jump phase encoding
gradients between the groups were combined to cover the whole k-space from the
center to the periphery.
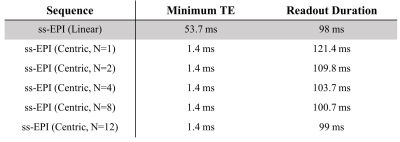
Fig. 2. Comparison of minimum
TE and readout duration between linear and proposed pseudo-centric EPI. Data acquisition parameters were as follows:
matrix size = 128*96, receiver bandwidth = 1560 Hz/px. Minimum TE was
significantly reduced with centric reordering compared to linear ordering. The
readout duration was elongated a bit with centric ordering compared to the
linear, but the difference was within 6% in general condition (N>2) and 20%
for dramatic condition (N=1).
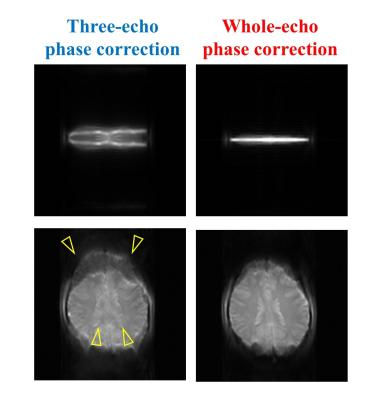
Fig. 3. Investigation on the
effect of phase correction scheme on single-shot pseudo-centric EPI. Single-shot pseudo-centric
EPI images without phase encoding gradients (Top, navigator data) and with phase encoding gradients (Bottom). Comparing the conventional
EPI phase correction using three echoes (left) and proposed whole echo correction (right), the image distortion and signal dropout due to the phase
errors shown in the left (indicated by yellow arrows) were reduced after whole
echo phase correction.
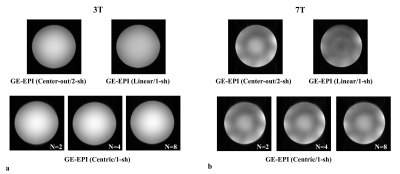
Fig. 4. Doped water phantom EPI
images acquired with various acquisition methods at 3T and 7T. (a)
3T GE-EPI images with 2-shot center-out reordering (Top, left), single-shot
linear reordering (Top, right), proposed single-shot centric reordering (Bottom, N=2,
4, and 8) (b) 7T GE-EPI images with 2-shot center-out reordering (Top,
left), single-shot linear reordering (Top, right), and proposed single-shot
centric reordering (Bottom, N=2, 4, and 8).
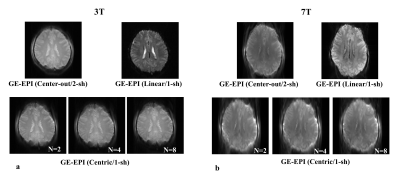
Fig. 5. Representative human
brain EPI images acquired with various acquisition methods at 3T and 7T. (a)
3T GE-EPI images with 2-shot center-out reordering (Top, left), single-shot
linear reordering (Top, right), proposed single-shot centric reordering (Bottom, N=2,
4, and 8) (b) 7T GE-EPI images with 2-shot center-out reordering (Top,
left), single-shot linear reordering (Top, right), and proposed single-shot
centric reordering (Bottom, N=2, 4, and 8).