3731
Highly Accelerated EPI with Wave Encoding and Multi-shot Simultaneous MultiSlice Imaging1Radiology, Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3College of Optical Science and Engineering, Zhejiang University, Hangzhou, China, 4Siemens Medical Solution, Boston, MA, United States, 5Electrical and Electronics Engineering, Bogazici University, Istanbul, Turkey, 6Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, United States
Synopsis
We combine wave controlled aliasing strategy with echo-planar imaging (EPI) and simultaneous multi-slice (SMS) encoding to increase acquisition efficiency and reduce geometric distortion simultaneously for functional (fMRI) and diffusion (dMRI) imaging. Wave-EPI enables RinxRsms=4x3-fold accelerated single-shot gradient-echo (GE)-EPI by reducing the maximum g-factor noise amplification by 2-fold compared to blip-CAIPI. We extend wave-EPI to multi-shot acquisition and incorporate Hankel low-rank constraint to leverage the similarities among the shots for improved reconstruction. This allows us to create a 10-second structural scan with 12-fold reduced distortion and push the acceleration rate to RinxRsms=6x3-fold in dMRI using 3-shots with markedly improved image quality.
Introduction
Wave controlled aliasing in parallel imaging (CAIPI) employs extra sinusoidal gradient modulations during the readout to harness coil sensitivity variations in all three-dimensions and achieve higher accelerations (1,2). Wave encoding was applied to GE-EPI to reduce B0-related distortion and blurring artifacts by providing higher acceleration (3,4), however the amount of voxel spreading has been limited by the small wave amplitude that could be played out during the short readout window.An alternative approach for mitigating distortion and blurring is multi-shot EPI, where MUSSELS algorithm (5) jointly reconstructs the shots using Hankel structured low-rank constraint to provide robustness against shot-to-shot phase variations. SMS encoding has also been widely used in EPI to reduce the imaging time and have also been combined with multi-shot EPI to speed up its acquisition (6).
In this abstract, we double the amount of voxel spreading that can be achieved by using “half-cycle” sinusoidals, and extend this optimized wave readout to GE-, SE- and diffusion-EPI and demonstrate up to 2-fold improvement in maximum g-factor. We further combine it with multi-shot SMS to push the limits of acceleration in structural and diffusion imaging.
Methods
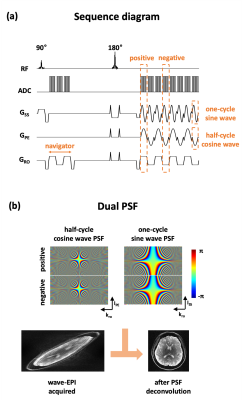
Figure 1a shows the proposed wave-EPI sequence diagram. The half-cycle of the cosine and the one-cycle of the sine wave-encoding gradients were applied in the phase-encoding and the slice-selective directions, respectively. This creates the usual “corkscrew” wave trajectory, but doubles the amplitude in the phase-encoding sinusoidal in the presence of stringent slew rate limitations during the short readout window.Due to the presence of gradient timing differences in all 3 axes, we propose to use different wave-PSFs to deconvolve the even and odd lines of EPI (Figure 1b). To estimate the experimental wave-PSF, we developed a reference scan based on FLEET (7) with and without wave-encoding. Reference scans were acquired twice with opposite readout gradient-polarity (8) to estimate the wave-PSF in both of positive and negative readout-gradient polarities. We fit the wave-PSF based on the sparse-frequency PSF model approach (9) as follows.
$$ \sigma = \operatorname*{argmin}_\sigma \| \mathscr{F}_y^{-1} P(\sigma) \mathscr{F}_y k_{no} - k_{wav} \|_2^2 $$
where $$$\sigma$$$ is the sparse-frequency variable, $$$P$$$ is the wave-PSF function, $$$k_{no}$$$ is the k-space reference data without the wave-encoding, and $$$k_{wav}$$$ is the k-space reference data with the wave-encoding, respectively.
The reconstruction of the wave-epi with low-rank constraint between multiple shots is described as follows.
$$\ I = \operatorname*{argmin}_I \sum_s \| \begin{bmatrix}W_p \mathscr{F}_y P(\sigma_p) \\ W_n \mathscr{F}_y P(\sigma_n) \end{bmatrix} \mathscr{F}_x C I_{s} - \begin{bmatrix}k_p \\ k_n\end{bmatrix} \|_2^2 +\| \mathscr{H}(I) \|_1 $$
where $$$I_s$$$ is the reconstructed image of $$$s$$$-th shot, $$$p$$$ represents the positive line, $$$n$$$ represents the negative line, $$$W$$$ is the SMS encoding with sub-sampling, $$$C$$$ is the coil sensitivity, $$$k$$$ is the acquired wave-EPI k-space data, and $$$\mathscr{H}$$$ is the Hankel matrix, respectively.
Three different experiments highlighting the versatility of wave-EPI were conducted on a 3T Siemens Skyra system with a 32-channel head coil.
Single-shot GE-EPI
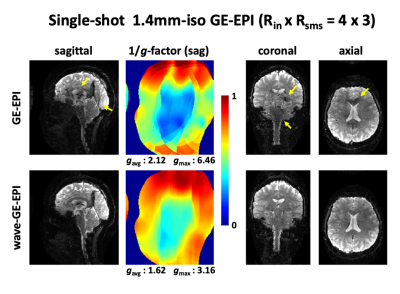
1.4mm-iso resolution, RinxRsms=4×3, TR is 5200ms, TE of GE-EPI is 40ms, TE of wave-EPI is 43ms, sagittal-slices, and 38mT/m x 19mT/m of the maximum wave-gradients were applied in the phase-encoding and slice-select directions.
Three-shot SE-EPI
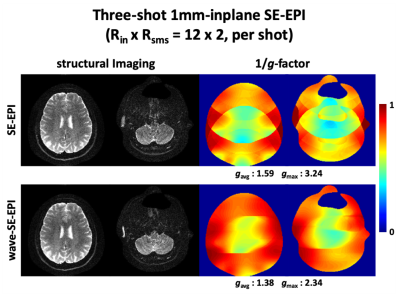
1x1x3mm3 resolution, RinxRsms=12×2 per shot, TR is 3300ms, TE is 95ms, axial-plane, and 18mT/m x 18mT/m of the maximum wave-gradient were applied in the phase-encoding and slice-select directions.
Two-shot SE-EPI DWI
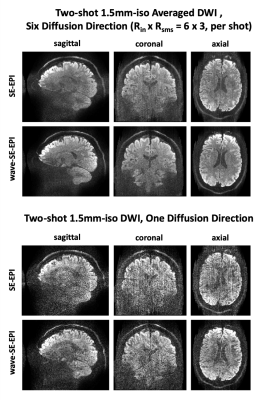
1.5mm-iso resolution, RinxRsms=6×3 per shot, TR is 5700ms, TE of SE-EPI is 65ms, TE of wave-EPI is 68ms, b-value 1000s/mm2 of diffusion gradients were applied in the six direction, sagittal-plane, and 38mT/m x 19mT/m of the maximum wave-gradient were applied in the phase-encoding and slice-select directions.
Results
Figure 2 shows single-shot 1.4mm-iso whole-brain GE-EPI images with RinxRsms=4x3. Using wave-encoding, averaged and maximum g-factors reduced by 1.3- and 2-fold, respectively. As can be observed, GE-EPI without wave-encoding suffers from severe artifacts from the high accelerations, that wave-encoding is shown to largely mitigate.Figure 3 shows three-shot 1mm-inplane SE-EPI structural images. Using wave-encoding, averaged and maximum g-factors reduced by 1.15- and 1.38-fold, respectively. The center of the SE-EPI results looks noisy, where the g-factor gets close to its maximum, while the wave-SE-EPI helps clean up the noise.
Figure 4 shows two-shot 1.5mm-iso SE-EPI DWI images with 1000s/mm2 of b-value in six directions. MUSSELS with 7x7 kernel block was used to mitigate the shot-to-shot phase variations. Wave-EPI significantly reduced artifacts despite the extreme acceleration level.
Discussion & Conclusion
Wave-EPI reduced g-factor noise amplification significantly by incorporating extreme controlled aliasing into multi-shot SMS EPI. Advanced hardware systems with relaxed slew and nerve stimulation constraints (e.g. head-insert gradient) will allow for yet higher acceleration.Wave-EPI cause 3 ms increase in the TE because of the rephasing lobe of the wave gradient. Employing ramp sampling will further decrease TE, which makes the difference of 10% of echo-spacing time. The proposed reference PSF calibration scan is flexible enough to allow for incorporating ramp sampling to provide this additional efficiency gain.
Acknowledgements
No acknowledgement found.References
1. Bilgic B, Gagoski BA, Cauley SF, et al. Wave-CAIPI for highly accelerated 3D imaging. Magnetic Resonance in Medicine 2015;73:2152–2162.
2. Gagoski BA, Bilgic B, Eichner C, et al. RARE/turbo spin echo imaging with simultaneous multislice Wave-CAIPI: RARE/TSE with SMS Wave-CAIPI. Magnetic Resonance in Medicine 2015;73:929–938.
3. Poser BA, Bilgic B, Gagoski BA, et al. Echo-planar imaging with wave-CAIPI acquisition and reconstruction. In: Proceedings of the 25th Scientific Meeting of ISMRM. Honolulu; 2017. p. 1198.
4. Cho J, Park H, Setsompop K, Bilgic B. Multi-shot Echo-planar Imaging with Simultaneous MultiSlice Wave-Encoding. In: Proceedings of the 27th Scientific Meeting of ISMRM. Montreal; 2019. p. 4768.
5. Mani M, Jacob M, Kelley D, Magnotta V. Multi-shot sensitivity-encoded diffusion data recovery using structured low-rank matrix completion (MUSSELS): Annihilating Filter K-Space Formulation for Multi-Shot DWI Recovery. Magnetic Resonance in Medicine 2017;78:494–507.
6. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magnetic Resonance in Medicine 2012;67:1210–1224.
7. Polimeni JR, Bhat H, Witzel T, et al. Reducing sensitivity losses due to respiration and motion in accelerated echo planar imaging by reordering the autocalibration data acquisition: Reducing Losses in Accelerated EPI with FLEET-ACS. Magnetic Resonance in Medicine 2016;75:665–679.
8. Hoge WS, Polimeni JR. Dual-polarity GRAPPA for simultaneous reconstruction and ghost correction of echo planar imaging data. Magnetic Resonance in Medicine 2016;76:32–44.
9. Cauley SF, Setsompop K, Bilgic B, Bhat H, Gagoski B, Wald LL. Autocalibrated wave-CAIPI reconstruction; Joint optimization of k-space trajectory and parallel imaging reconstruction. Magnetic Resonance in Medicine 2017;78:1093–1099.
Figures