3721
Multiband accelerated Fast Interrupted Steady-State (FISS) imaging with 2D Cartesian sampling1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
In this work we demonstrate the application of Fast Interrupted Stead-State (FISS) imaging with 2D Cartesian sampling and multiband (MB) acceleration to regain scan efficiency. MB-FISS cardiac cine imaging and a free-running sequence for vessel imaging are shown to work reliably with good fat suppression. For cine cardiac imaging FISS with readouts equal to number phase-encode lines in each cardiac segment had minimal scan time increase, while FISS (n=1) lead to breath-hold durations increase ~2.5 fold, but benefit from improved fat suppression and no additional ghosting artefacts compared to bSSFP.
Introduction
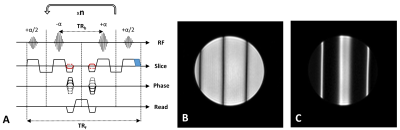
The recently proposed fast interrupted steady-state (FISS) sequence was first demonstrated with 2D radial sampling for cine cardiac imaging and, combined with ASL, to visualise blood flow1,2. More recently 3D cardio-respiratory resolved imaging of the heart has been demonstrated with both radial3 and Cartesian sampling4.The FISS sequence has been shown to offer similar signal and contrast to conventional balanced steady-state free precession (bSSFP) for on-resonance signal, but can also yield a large stop-band in the frequency response which can be exploited for effective fat suppression, additional benefits include reduced flow artefacts and off-resonance signal instabilities. However, the method inevitably increases scan time, by ~2.5x for the case of one readout per FISS module (n=1, see Fig.1). Although multiple readouts per module have been used effectively with radial sampling, this can lead to ghosting artefacts in Cartesian imaging due to signal fluctuation within the FISS module, in addition to narrowing the usable stop-band for fat suppression.
Here we demonstrate FISS imaging with 2D Cartesian sampling and use multiband (MB) acceleration to regain scan efficiency in multi-slice imaging, and apply to a widely-used retrospective gated cine sequence in the heart, and also to vessel imaging in the leg using a free-running multi-dynamic sequence.
Methods
The FISS technique was implemented along with blipped-CAIPI multiband as previously described for cine bSSFP5 on a 3T Philips Achieva system. The FISS sequence employed RF spoiling (117°) between each module, and variable gradient spoiler duration - in most cases this was kept to the minimum required for the α/2 slice selection to avoid extending the shot duration. MB factors up to 4 were tested with FISS and conventional bSSFP for reference.For cardiac cine imaging two FISS operating modes were implemented with a 2D Cartesian retrospective gated sequence: 1) FISS with n=TFE factor (phase-encode lines per segment), determined by the R-R interval / number of cardiac phases / TRb - (typically: 14-17), and 2) FISS with n=1 leading to a reduced TFE factor = R-R/cardiac phases/TRf (typically: 6-7). Standard parameters for short axis stack analysis were used: flip angle= 40-45°, TRb=2.8-3.6ms, TE=TR/2, acquired resolution=2x1.6x8mm, 30 cardiac phases (64% minimum acquired before temporal interpolation, partial-Fourier=80%), in vivo data was acquired from a healthy adult male volunteer using a 32-channel cardiac coil and vector-cardiogram gating.
A free-running bSSFP and FISS (n=1) sequence was also tested on two healthy female volunteers using a Transmit-Receive knee coil (16-channel receive) and 8-channel extremity coil. Imaging parameters: flip angle=40°, resolution=1.4x1.4x4mm, TRb=3.4, TE=TR/2, 10 dynamics, MB=1-4.
Multiband reconstruction was performed with custom scripts (Matlab) and MRecon (GyroTools, Zurich).
Results and Discussion
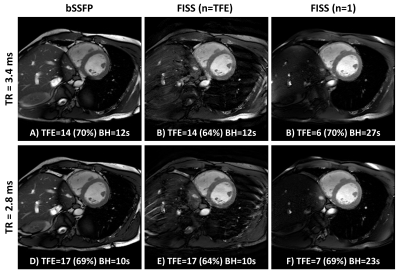
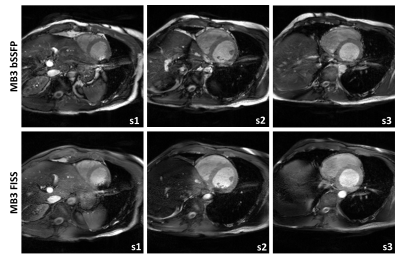
2D Cartesian FISS cine imaging is presented in Fig.2 for both modes of operating (n=TFE and n=1), alongside a conventional bSSFP scan. For the TRb=3.4ms case, FISS (n=TFE) has performed well with good fat suppression and relatively minor ghosting artefacts, whereas for minimum TRb=2.8ms the ghosts are more prominent at the periphery. In both cases the small extension of the TFE shot due to extra α/2 tip down and restore interruptions have only reduced the acquired phase percentage by 5-6% whilst maintaining the same breath-hold duration. For FISS (n=1) the TFE factor drops from 14 to 6 and 17 to 7, resulting in a scan time increase from 12 to 27, and 23 seconds, respectively, for each of the TRs shown. However, there is a clear benefit of improved fat suppression and no issues from ghosting or eddy current artefacts.In Fig.3 MB3 accelerated bSSFP and FISS cine data are shown. Here, due to operating close to the SAR limits, adding multiband has led to a small increase in TR=3.6ms and with MB FISS (n=1) the minimum TRb=3.3ms leading to a dark-band encroaching the myocardium in the most apical slice. Incidentally, it appears the bright signal enhancement in the adjacent blood is less hyper-intense and more stable in FISS compared to bSSFP. The residual multiband leakage artefacts in MB-FISS (n=1) are lower than in MB-bSSFP, probably due to fat saturation.
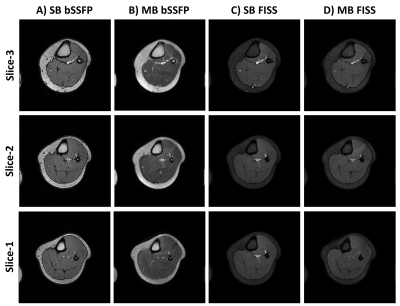
In Fig.4 we demonstrate multiband accelerated FISS imaging in the leg and compare to bSSFP acquisitions. Due to the near 4x increased in the SAR limits for extremity imaging, MB acquisitions can be acquired with negligible scan time or TR penalty, in addition to the ability to shim the volume of interest without the presence of the air-tissue interfaces which make cardiac imaging at 3T challenging - even the on-resonance band-pass for FISS n=1 is sufficient over the whole volume.
Conclusion
In this work we demonstrate the application of multiband acceleration to FISS imaging with Cartesian sampling. For cine cardiac imaging we test two modes of operating FISS: n=1 and n=TFE (determined by the imaging parameters and heart rate). Results showing good fat suppression can be achieved with FISS n=TFE even with Cartesian sampling. However, the ghost artefacts are both parameter and B0 shim dependant. MB-FISS (n=1) has been shown to work reliably due to the excellent fat suppression and immunity to ghosting artefacts that can affect n>1, whilst the application to cardiac imaging at 3T is challenging due to banding artefacts, at lower field and in other anatomic regions, like the extremities, MB-FISS can be used without significant penalty.Acknowledgements
This work is supported by the Wellcome EPSRC Centre for Medical Engineering at Kings College London (WT 203148/Z/16/Z) and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
1. Koktzoglou. MRM (2018) 79:2077-2086.
2. Edelman et al. JCMR (2018) 20:12.
3. Bastiaansen et al. MRM (2020) 83:45-55.
4. Küstner et al. MRM (2019) 82:1617-1630.
5. Price et al. MRM (2019) DOI: 10.1002/mrm.28086
Figures