3718
Clinical valid of TOF-MRA with sparse under-sampling in evaluation of intracranial aneurysm: DSA as a reference standard as a reference standard1Department of Radiology, West China Hospital, Sichuan University, Chengdu, China
Synopsis
When compared with conventional TOF, the sparse TOF which applied compressed sensing to accelerate the acquisition time has been proved to be well-performed in evaluation of UIAs. However, the image quality and the agreement between sparse TOF and DSA need to be studied further. We therefore assessed the clinical validation of sparse TOF compared with conventional TOF in qualitative and quantitative image qualities and explored the correlation among two MRAs and DSA in evaluation of size parameters.
Introduction
Before rupture, UIAs are often found incidentally without obvious positive signs. Considering aneurysm geometry plays a key role in treatment planning, high spatial resolution images are expected to provide accurate detection and assessment of UIAs. At present, the invasive and expensive method DSA is commonly considered to be the reference-standard technique. And considering the advantages of being a noninvasive, contrast-free MRA technique TOF-MRA is favored in initial evaluation. However, high spatial resolution and large coverage images require relatively long acquisition times in it. Recently, the innovative sparse recovery framework compressed sensing (CS) is widely applied in MRA to accelerate the imaging acquisition. In previous studies, compared with TOF-MRA the clinical valid of sparse TOF in UIAs has been proved to be excellent. But the reference standard DSA has not be considered in those studies yet. The aim of this study is to compare the image quality between sparse TOF and con-TOF, and explore the correlation between DSA and sparse TOF.Method
A total of 46 consecutive adult patients with suspected UIAs were prospectively enrolled into this study. All MRA examinations were performed on a 3.0 T MR scanner (Skyra, Siemens Medical Systems, Erlangen, Germany) using a 20-channel head-neck coil. And All patients underwent DSA examination after MRAs within one week. For objective image quality, the slices with the largest cross section of UIAs were selected on axial images of MRAs, and obtained the signal intensity (SI) of the aneurysms, SI of adjacent brain tissue and the background noise. For each patients, the contrast-to-noise ratio (CNR) and signal-to-noise (SNR) of two MRA images were calculated by the equations: SNR= SIaneurysm/SD, CNR= (SIaneurysm- SIbrain)/SD. The subjective image quality was assessed using a 5-point score scale by by two experienced neuroradiologists (with 7 and 26 years of experience) who were blinded to clinical findings and DSA results using a five-point grading subjectively and the interobserver agreement was determined. With DSA as the reference, the neck, height and width of aneurysms in two MRAs were measured and compared on maximum intensity projection (MIP) image data sets respectively. The Kolmogorov-Smirnov test was used to test the normality of the variables. Statistical significance was defined as P < 0.05.Results
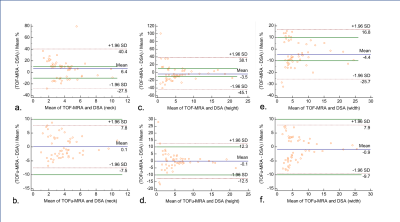
When regarding overall image quality, sparse TOF showed comparable image quality (4.83±0.376 vs. 4.48±0.574, p<0.05) compared with con-TOF. Excellent interobserver agreement (к values=0.94) was obtained between the two radiologists. For quantitative measurement, the SNR and CNR in sparse TOF (SNR = 24.21±8.06; CNR =17.08±7.44) were significantly higher than those in con-TOF (SNR = 18.94±6.44; CNR = 13.27±6.00) (P < 0.001). For measurement of size parameter, there were significant differences between con-TOF and sparse TOF among three groups (neck, 3.63±2.22 vs. 3.52±2.16; width, 4.62±4.59 vs. 4.64±4.71; height, 4.62±4.59 vs. 4.64±4.71, all P<0.05). And there were no significant differences between sparse TOF and DSA in measurement of neck, height and width of UIAs. In the Bland-Altman plot analysis (Fig.1), sparse TOF showed no significant differences between DSA (with a mean difference of 0.1mm, -0.9mm and -0.1mm for neck, height and width respectively).Discussion
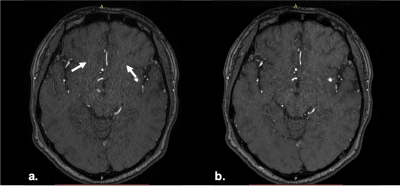
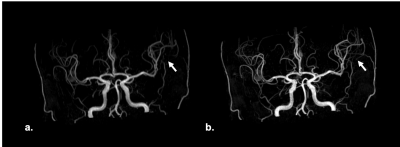
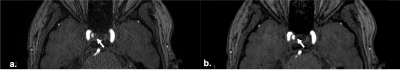
Different to the conventional TOF, the acceleration factor of sparse TOF is affected by the undersampling factor and the SNRs of reconstructed images also correlated with undersampling and the iteration steps. Therefore, with the same phased array coils TOFu can use a higher acceleration factor. We found that the PI ghost artifacts were reduced by CS techniques (Fig.2). Further, the delineation of small vessels in TOFu-MRA images was improved (Fig.3). In addition, the edge of UIAs in sparse TOF images were found shaper than con-TOF’s in our study (Fig.4).Conclusion
Compared with conventional TOF, sparse TOF could assess UIAs more accurately with a better image quality.Moreover, our study showed a strong agreement between TOFu-MRA and DSA when assessed size parameter of aneurysms. That indicated that TOFu-MRA could potentially be used to achieve effective prediction and treatment of UIAs.Acknowledgements
The authors acknowledge Jinge Zhang and Lingming Zeng for their great support.References
[1] Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown 7. RD, Piepgras DG, et al. Unruptured intracranial aneu- rysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–10.
[2] UCAS Japan Investigators, Morita A, Kirino T, Hashi K, 8. Aoki N, Fukuhara S, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366:2474–82.
[3] Ellis J A , Nossek E , Kronenburg A , et al. Intracranial Aneurysm: Diagnostic Monitoring, Current Interventional Practices, and Advances[J]. Current Treatment Options in Cardiovascular Medicine, 2018, 20(12).
[4] Liu H, Xu Y , Xun Y , et al. Diagnostic value of 3D time-of-flight magnetic resonance angiography for detecting intracranial aneurysm: a meta-analysis[J]. NEURORADIOLOGY, 2017, 59(3):1083-1092.
[5]Lin FH, Kwong KK, Belliveau JW, Wald LL. Parallel imaging reconstruction using automatic regularization. Magn Reson Med 2004;51:559-567.
[6]Michaely HJ, Herrmann KA, Kramer H, Dietrich O, Laub G, Reiser MF, et al. High-resolution renal MRA: comparison of image quality and vessel depiction with different parallel imaging acceleration factors. J Magn Reson Imaging 2006;24:95-100.
[7] Stalder AF, Schmidt M, Quick HH, Schlamann M, Maderwald S, Schmitt P, et al. Highly undersampled contrast-enhanced MRA with iterative reconstruction: integration in a clinical setting. Magn Reson Med 2015;74:1652-1660.
[8] Yoon JH, Lee SM, Kang HJ, et al. Clinical feasibility of 3-dimensional magnetic resonance cholangiopancreatography using compressed sensing: Comparison of image quality and diagnostic performance. Invest Radiol 2017;52:612–619.
[9] Garwood ER, Recht MP, White LM. Advanced imaging techniques in the knee: Benefits and limitations of new rapid acquisition strategies for routine knee MRI. AJR Am J Roentgenol 2017;209:552–560.
[10] Wetzl J, Lugauer F, Schmidt M, Maier A, Hornegger J, Forman C. Free‐breathing, self‐navigated isotropic 3‐D CINE imaging of the whole heart using Cartesian sampling. Paper presented at: Proceedings of the 24th International Society for Magnetic Resonance in Medicine; 2016:Singapore.
[11] Nam S, Hong SN, Akcakaya M, et al. Compressed sensing reconstruction for undersampled breath‐hold radial cine imaging with auxiliary free‐breathing data. J Magn Reson Imaging. 2014;39:179‐188.
[12] Tang H , Hu N , Yuan Y , et al. Accelerated Time-of-Flight Magnetic Resonance Angiography with Sparse Undersampling and Iterative Reconstruction for the Evaluation of Intracranial Arteries[J]. Korean Journal of Radiology, 2019, 20(2).
[13] Lin Z , Zhang X , Guo L , et al. Clinical feasibility study of 3D intracranial magnetic resonance angiography using compressed sensing[J]. Journal of Magnetic Resonance Imaging, 2019.
[14] Numminen J, Tarkiainen A, Niemela M (2011) Hernesniemi J, Kangasniemi M. Detection of unruptured cerebral artery aneurysms by MRA at 3.0 tesla: comparison with multislice helical computed tomographic angiography. Acta Radiologica 52:670–674.
[15] International Study of Unruptured Intracranial Aneurysms Investigators (1998) Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. N Engl J Med 339: 1725–1733.
[16] Greving JP, Wermer MJ, Brown RD Jr, Morita A, Juvela S, Yonekura M, Ishibashi T, Torner JC, Nakayama T, Rinkel GJ, Algra A (2014) Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol 13:59–66.
[17] Wiebers DO, Whisnant JP, Huston J 3rd, et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103‐110.
[18] Dhar S, Tremmel M, Mocco J, Kim M, Yamamoto J, Siddiqui AH, et al. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery 2008;63(2):185–97.
[19] Morita A, Tominari S. Abstract 14: size ratio can be a strong predictor for future rupture of the unruptured cerebral aneurysms. Stroke 2016;47(Suppl. 1):A14.
[20] cankaowanxian1[21] Kim H J , Yoon D Y , Kim E S , et al. Intraobserver and interobserver variability in CT angiography and MR angiography measurements of the size of cerebral aneurysms[J]. Neuroradiology, 2017, 59(5):491-497.
Figures