3717
Real-Time Z-Shimming for Magnetic Resonance Imaging of the Spinal Cord
Eva Alonso-Ortiz1, Cyril Tous2,3, Ryan Topfer1, and Julien Cohen-Adad1,4
1NeuroPoly Lab, Ecole Polytechnique, Montreal, QC, Canada, 2Department of Radiology, Radiation-Oncology and Nuclear Medicine and Institute of Biomedical Engineering, Université de Montréal, Montreal, QC, Canada, 3Laboratory of Clinical Image Processing Le Centre de Recherche du CHUM, Centre hospitalier de l'Université de Montréal, Montreal, QC, Canada, 4Functional Neuroimaging Unit, Centre de recherche de l'Institut universitaire de gériatrie de Montréal, Montreal, QC, Canada
1NeuroPoly Lab, Ecole Polytechnique, Montreal, QC, Canada, 2Department of Radiology, Radiation-Oncology and Nuclear Medicine and Institute of Biomedical Engineering, Université de Montréal, Montreal, QC, Canada, 3Laboratory of Clinical Image Processing Le Centre de Recherche du CHUM, Centre hospitalier de l'Université de Montréal, Montreal, QC, Canada, 4Functional Neuroimaging Unit, Centre de recherche de l'Institut universitaire de gériatrie de Montréal, Montreal, QC, Canada
Synopsis
Spinal cord MRI is notoriously challenging, in part due to the fact the spinal cord is near the lungs, causing magnetic field inhomogeneities that are changing throughout respiration. We propose a novel solution, called real-time z-shimming to address this problem. Real-time z-shimming involves compensating for magnetic field inhomogeneities that arise during image acquisition, by adapting the sequence in real-time. Our findings show that real-time z-shimming can recover signal loss, caused by respiration-induced magnetic field inhomogeneities, in the spinal cord region.
Introduction
One of the main challenges to obtaining high quality images of the spinal cord is the fact that the spinal cord is close to the lungs, which cause artifacts due to the difference in magnetic susceptibility between air and tissue. During respiration, the volume of air in the lungs varies, in turn causing magnetic field (B0) variations that are changing throughout respiration and lead to image artifacts1. Previous studies introduced z-shimming for improving spinal cord acquisitions2, however this approach is static in that each slice is assigned a unique Gz value, regardless of respiratory state. Other work introduced a method to account for respiratory-related B0 variations3, however, this work required the use of a dedicated external shim coil hardware and hence is not practical. We propose a novel solution, real-time z-shimming, to correct for both static and dynamic B0 variations during image acquisition.Methods
We implemented real-time z-shimming within a 2D multi-gradient echo (MGRE) sequence (see the sequence diagram in Fig. 1) by adding “compensation” gradients along the z-axis, prior to every readout, with momentum equal to -Gz*TE, where Gz is the local field gradient along the slice-select direction (z) and TE is the echo time.A single volunteer was scanned on a Siemens 3T scanner with a 64-channel head-neck coil and a 48-channel spine coil. A respiratory bellow (Siemens) was placed on the volunteer’s chest to inform on the respiratory state, via pressure readings (as shown in Fig. 1). A series of single-slice sagittal B0 maps were first acquired along the midline of the spinal cord, using a B0 mapping sequence (TR=7.2ms, TE1=2.46ms, TE2=4.93ms, matrix=96x64, image resolution=3x3x3mm3, 60 repetitions). From the B0 images, a time series of Gz maps was generated so that the respiration-induced Gz changes could be determined by examining the linear relationship between the time-varying Gz maps and the respiratory cycle3,4. This results in a relationship between Gz(t) and pressure, P(t):
Gz(t) = Gz,static + c(z)*P(t) [Eq.1]
A short axial GRE scan (without shimming) was also acquired for the purpose of automatic spinal cord segmentation using SCT5, so that average Gz(t) values could be obtained for the spinal cord region. Those values were then quickly transferred to the scanner via ethernet socket and MGRE images with real-time z-shimming were acquired (TR=1000 ms, 6 echoes with 3ms inter-echo spacing, TE1=2.5ms, BW = 600 Hz/px, 20 slices, matrix=128x68, image resolution=2.2x2.2x3mm3). During the shimmed acquisition, the pressure from the respiration bellow was sampled prior to each slice acquisition and the appropriate compensation gradient was calculated (according to Eq. 1).
Results
Real-time z-shimming was found to recover signal loss in the spinal cord. In Fig. 2 we show sagittal and axial views of the spinal cord with and without real-time z-shimming. The red arrow indicates areas in which real-time z-shimming recovered some of the signal that is lost due to respiration-induced B0 field inhomogeneities.Discussion and Conclusions
We introduced a novel approach that combines z-shimming with real-time update from respiration trace, for compensating both static and dynamic B0 variations in the spinal cord. Our approach does not require additional software, making its translation to other centers straightforward. The proposed method will help make MRI techniques that are sensitive to magnetic field inhomogeneities, such as DTI, functional MRI and spectroscopy more feasible in the spinal cord. We expect the gain to be even larger at ultra-high field, which will be the purpose of follow-up studies.Acknowledgements
Funded by the Canada Research Chair in Quantitative Magnetic Resonance Imaging [950-230815], the Canadian Institute of Health Research [CIHR FDN-143263], the Canada Foundation for Innovation [32454, 34824], the Fonds de Recherche du Québec - Santé [28826], the Fonds de Recherche du Québec - Nature et Technologies [2015-PR-182754], the Natural Sciences and Engineering Research Council of Canada [RGPIN-2019-07244], the Canada First Research Excellence Fund (IVADO and TransMedTech), the Courtois NeuroMod project and the Quebec BioImaging Network [5886, 35450].References
- Verma and Cohen-Adad, Magnetic Resonance in Medicine 72:1629–1636 (2014)
- Finsterbusch et al., Neuroimage 79:153-61 (2013)
- Topfer et al., Magnetic Resonance in Medicine 80:935–946 (2018)
- Vannesjo, et al., Magnetic Resonance in Medicine 81(6):3745–3753 (2019)
- De Leener et al., Neuroimage 15;145(Pt A):24–43 (2017)
Figures
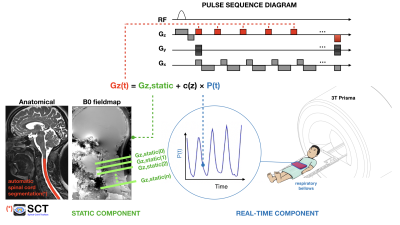
Figure 1: Workflow for real-time z-shimming. First, the respiration trace is recorded and a series of short-TR dual echo single-slice GRE scans are acquired. A short axial GRE scan is also acquired for the purpose of automatic spinal cord segmentation using SCT5. Data are transferred to a laptop using an ethernet socket, optimal Gz values are estimated as a function of slice and respiratory pressure, and these info are sent back to the scanner to be read by the sequence.
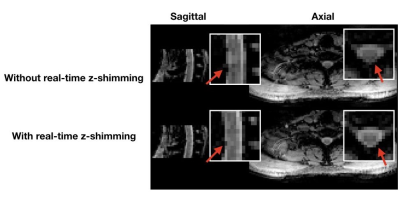
Figure 2: Sagittal and axial MGRE magnitude (TE4 = 11.5 ms) images are shown with and without real-time z-shimming. The red arrow indicates areas in which signal loss was recovered. Image scaling is the same.