3716
4D-EPICS:Compressed Sensing EPI for highly accelerated fMRI at 7T
1Spinoza Centre for Neuroimaging, Amsterdam, Netherlands, 2Delft University of Technology, Delft, Netherlands, 3Radiology and Nuclear Medicine, Amsterdam UMC, Amsterdam, Netherlands, 4Cognitive Psychology, Vrije Universiteit Amsterdam, Amsterdam, Netherlands, 5Maastricht Brain Imaging Centre, Faculty of Psychology and Neuroscience, Maastricht University, Maastricht, Netherlands
Synopsis
The proposed EPICS readout takes a traditional EPI readout and makes it suitable for Compressed Sensing. This novel readout keeps the EPI direction constant and employs pseudo-spiral under-sampling on a Cartesian grid in the other two (phase encoded) directions. EPICS delivers the flexibility offered by spiral sequences with the relative ease of Cartesian CS reconstruction. Multiple spiral types and patterns with flexible TE's are compared. In-vivo brain image data shows good image quality at 7T. Future 4D-EPICS fMRI acquisitions will be able to profit from regularized CS reconstructions.
Introduction
Echo-planar Imaging (EPI) is a very efficient and fast readout strategy and is therefore often used in functional and diffusion imaging. More recently Compressed Sensing (CS)1 has allowed acceleration of many scan types, but for Cartesian EPI the required pseudorandom sampling patterns are difficult to create. Spiral and radial EPI readouts are more suitable for CS, but are more difficult in practice due to gradient imperfections, that often require correction using field monitoring2. The here proposed Echo-Planar Imaging Compressed Sensing (EPICS) readout keeps the EPI direction constant and allows for spiral under-sampling on a Cartesian grid in the two phase encoding directions.Expected benefits of functional MRI using EPICS will consist of (a) increased SNR by more often sampling the centre k-space (b) increased flexibility in creating a time-series with varying temporal resolution/image quality and (c) the possibility to regularize in the sparse 4th dimension.
Methods
Five healthy volunteers were scanned on a Philips Achieva 7T system (Best, Netherlands) with Nova Medical 32Rx/8Tx coil (Wilmington, USA). All gave written consent. All scans were performed with a FOV of 202x202x176mm3, matrix=112x112x98 and 1.8mm3 voxel size and EPI direction head-feet.Three types of spirals have been compared: outside-in, inside-out and outside-in-out. All sampling patterns are constructed by placing the EPI readout positions on a Cartesian gridded 180° spiral and repeating these pseudo-spiral EPI shots by rotating the ungridded spiral with a golden angle and randomly jittering the position of the sample points to improve the randomness of the distribution. An exemplar sampling pattern is depicted in figure [1]. The resulting pulse sequence diagram of an EPICS shot is depicted in figure [3].
The outside-in spiral has an EPI factor of 27, with TR/TE/α = 34ms/20ms/13°. The inside-out spiral also used an EPI factor of 27, with TR/TE/α = 34ms/0ms/13°. Since the arms of the outward spiral start in the center the effective echo-time is close to zero. The outside-in-out spiral is a combination of the outside-in and inside-out spirals in succession. The EPI factor is raised to 55 and this results in TR/TE/α = 52/20/13°. The drawback of this spiral is that the amount of T2* weighting of the acquired lines is not smooth near the sides of K-space, since the EPI readout alternately both begins and ends near the outside of K-space.
As a reference for the EPICS data, an normal Cartesian 3D-EPI with the same duration was used. This reference scan uses SENSE 2.2x2.4, an EPI factor of 51 and TR/TE/α = 50ms/20ms/13°. An entire volume is collected every 2 seconds.
The raw data was acquired and pre-processed using the ReconFrame platform (Gyrotools, Zurich). The reconstruction pipeline uses the BART toolbox3 to both calculate the coil sensitivity maps and perform the reconstruction with L1-wavelet regularization in image space and total variation regularization in time.
Results
The three spirals result in similar sampling distributions. Even though all sampling positions are randomly jittered, there is still an slight spiral pattern visible. An exemplar distribution is depicted in figure [2].Exemplar outside-in spiral and reference EPI volumes are depicted in figure [4]. The contrast and SNR are comparable between both acquisition types, but the spiral suffers in regions with higher B0 inhomogeneities.
Discussion
Since the centre of K-space is traversed in both directions consecutively in every EPICS shot, it is possible to correct for EPI phase delays and B0 fluctuations every TR. The current processing pipeline has no support for this yet, but it will be implemented in future versions and is expected to further improve imaging results and especially in application to fMRI.All data is currently gridded to correct for the ramp sampling, but the phase encoding blips are not yet taken into account. As can be seen in figure [3], the blips are being played out during the sides of the acquisition and should thus be included in the future gridding process.
Due to computation-resource limitations, it is currently not yet possible to reconstruct an entire functional run of a spiral acquisition and therefore no functional analysis is performed.
Unlike regular EPI, the pseudo-spirals used in EPICS provide a high degree of flexibility on choosing the amount of T2* weighting. The outside-in spiral is great for BOLD since it gives BOLD at an echo time of choice. In other (f)MRI techniques, the short TE produced by the inside-out spiral is desirable to maximise SNR and reduce T2* weighting, such as in ASL and VASO sequeces.
An important aspect of the EPICS sequence is that since the center of K-space is acquired frequently and with the use of an Golden angle, it is possible to flexibly trade-off spatial resolution for temporal resolution. This can be for example used to obtain an HRF estimation and motion correction parameters.4
Conclusion
In conclusion, we have proposed 4D-EPICS as a versatile Cartesian echo-planar spiral hybrid that combines the flexibility offered by spiral sequences with the relative ease of Cartesian CS reconstruction. Next to the obvious application to BOLD fMRI, the ability to acquire at minimal TE further makes the sequence an attractive candidate for blood flow (CBF) and blood volume weighted (VASO) fMRI.Acknowledgements
Wim Prins, for his technical support on implementing the sequence on the scanner.
Royal Netherlands Academy of Arts and Sciences, for funding this work.
References
- Lustig, M. , et al. (2007), Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn. Reson. Med., 58: 1182-1195. doi:10.1002/mrm.21391
- Barmet, C. , et al. (2008), Spatiotemporal magnetic field monitoring for MR. Magn. Reson. Med., 60: 187-197. doi:10.1002/mrm.21603
- BART Toolbox for Computational Magnetic Resonance Imaging, DOI: 10.5281/zenodo.592960
- Graedel, N. et al. (2017), Motion correction for functional MRI with three‐dimensional hybrid radial‐Cartesian EPI. Magn. Reson. Med., 78: 527-540. doi:10.1002/mrm.26390
Figures
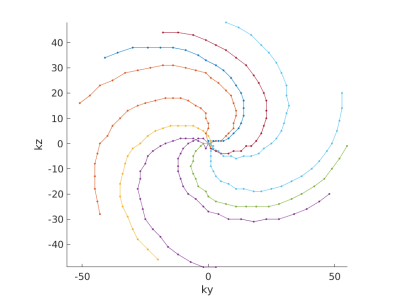
K-space sampling pattern of 10 outside-in spirals.
Each arm is one EPI shot with an EPI factor of 27. The biggest step size in an encoding direction is limited to 5 steps. The rotation within one arm is 180° and arms are repeated with golden angles (137.5°). All sampling points, except the center are randomly jittered by a maximum of 3 encoding steps. The center is always sampled twice consecutively, to traverse K0 in both EPI directions.
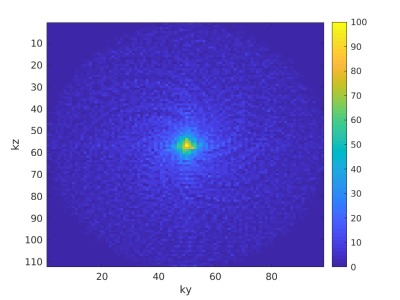
K-space sampling distribution from 60 seconds of outside-in spirals.
During 60 seconds of scanning, 1765 EPI shots with a length of 27 can be collected (TR = 34ms). The resulting 47655 sampling point coordinates are plotted here on their locations to show the resulting distribution. The variable density can clearly be seen by the higher amount of sampling points towards the center of K-space. Even though a very slight spiral pattern can be observed in the distribution, it is still very random.
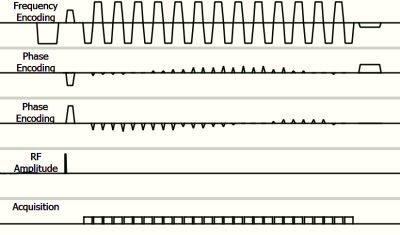
Pulse sequence diagram of outside-in spiral.
The 27 EPI readout segments are shown on the frequency encoding axis. The 26 blips in between the readout segments and the traversal to the outside of K-space are shown on the two phase encoding axes. The gap in between acquisitions is only 0.12ms and therefore the readouts are both during the ramp of the readout and also during the blips as the blips are 0.32ms long.
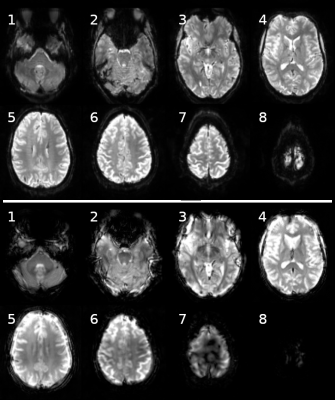
8 slices of outside-in spiral volume (bottom) and reference SENSE volume (top).
Both volumes are reconstructed from 2 seconds of samples. The SNR and contrast look quite similar between the two acquisitions types. The spiral scan suffers in regions where the B0 field is more inhomogenious, near the edges of the brain and in particular near the top of the brain.