3714
SPRING TSE: 2D T2-Weighted Brain Imaging using SPiral RING Turbo Spin-Echo1Biomedical Engineering, University of Virginia, CHARLOTTESVILLE, VA, United States, 2Radiology & Medical Imaging, University of Virginia, CHARLOTTESVILLE, VA, United States
Synopsis
2D Cartesian turbo spin-echo (TSE) is widely used in the clinical neuroimaging, yet the high specific absorption rate (SAR) induced by a large number of refocusing RF pulses limits its use in high magnetic field. Thus, this study describes a new TSE sequence with annular spiral ring acquisitions, dubbed “SPRING TSE”, for fast T2-weigthed imaging and reducing SAR. Preliminary results show that two sets of high spatial resolution images with intermediate and strong T2-weighted contrast characteristics can be obtained by the proposed method within a few seconds.
INTRODUCTION
Spiral trajectories have a number of benefits for fast imaging [1], including efficient k-space sampling and robustness to motion artifacts when compared to the conventional Cartesian sampling. Spiral acquisitions have been incorporated into a 2D TSE signal generation module via two strategies: an interleaved, rotated spiral-arm segmentation and an annular ring segmentation. The first requires a double encoding strategy [2] and a signal demodulation method to mitigate the swirl-like artifacts due to T2-decay induced signal variation, resulting in an increased scan time. The annular ring strategy [3, 4] splits long spiral trajectories into several annular segments, with the benefit of reduced T2 decay artifacts by converting the T2-dependent signal modulation into a k-space apodizing filter. However, the latter ring strategy has not been fully explored in either single excitation or multi-shot acquisitions. In this work, a new technique combining shared-view acquisition [5] with multi-shot spiral ring TSE is proposed to obtain high spatial resolution and two sets of T2-weigthed images within a short scan time.METHODS
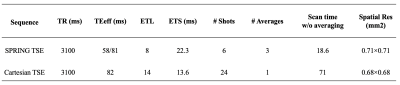
A schematic of the pulse sequence is shown in Figure 1. Spectral-spatial [6] RF water excitation pulses were used to suppress lipids, and 180° sinc RF pulses were utilized for refocusing. The first interleaved, black colored spiral arms were used for field map acquisitions as in Block [3] et al. After that, the center of k-space was sampled both at an early echo time and a late echo time by the first and second spiral rings, respectively, while the outer k-space regions were sampled by the same outer spiral rings. These green common echoes were then shared by both the orange and blue echoes, which allowed data for the short and long TE images to be acquired simultaneously.Spiral rings were designed by splitting the original constant density, multi-shot spiral-out arms into segments of equal time duration, each of which was played during one of the echoes in the TSE module. A model-based spiral trajectory measurement [7] was utilized to reduce the image blur and distortion induced by eddy current effects and other hardware imperfections, including the anisotropic timing delays on different physical axes. A simple center frequency offset correction was used to correct for off-resonance effects. Sequence parameters are given in Table 1.
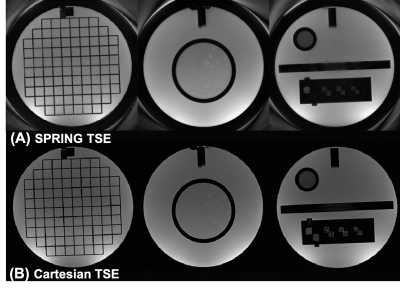
All experiments were performed on a 1.5T scanner (MAGNETOM Avanto, Siemens Healthcare, Erlangen, Germany) with a 12-channel head coil array. For both phantom and healthy volunteer studies, multiple slices with 4 mm thickness and 2 mm gap were imaged both by the proposed method and by conventional Cartesian TSE as a reference.
RESULTS AND DISCUSSION
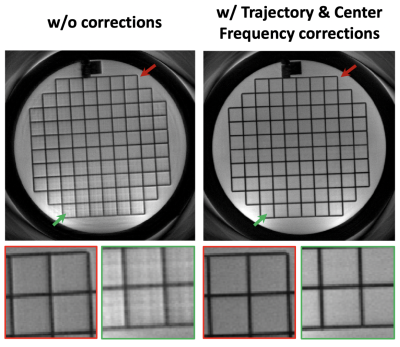
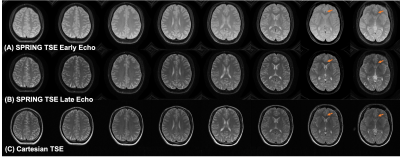
Figure 2 demonstrates the performance of both the spiral ring trajectory correction and the center frequency offset correction. Without correction, image distortion and strong off-resonance artifacts are present in the images. With trajectory correction, edge artifacts (red arrows) are reduced. By further performing the center frequency correction using the field map data, blurring artifacts (green arrows) are significantly reduced. Phantom and in-vivo results are shown in Figures 3 and 4, respectively. In the phantom study, only late echo images were acquired. In vivo results show that the image quality of the late echo images from the SPRING TSE method are, in general, comparable to that of Cartesian TSE, in terms of SNR and image contrast, with the addition of the early echo images. With three image averages, the total acquisition time for SPRING TSE is 55.8 s, which is still faster than that from Cartesian TSE without any averaging. In terms of SAR, even though in typical Cartesian TSE, the refocusing RF angle is reduced to 120°, the relative SAR from the refocusing RF pulses is approximately $$$(180^2 \ast 8)\ / (120^2 \ast 24) \sim75\%$$$ of the SAR of Cartesian TSE. However, signal loss was seen in some regions where there are strong susceptibility gradients, as shown in Figure 4, and ghosting artifacts, likely induced by concomitant gradients, were observed in sagittal and coronal views (not shown). Future work will focus on improved B0 inhomogeneity correction [8] and concomitant gradient correction.CONCLUSION
In this study, 2D T2-weighted brain imaging using spiral ring TSE was implemented and tested. The results show that within several seconds, the proposed method achieves 15-slice, dual-contrast T2-weighted images at 0.71 x 0.71 x 4 mm3 spatial resolution per slice and with lower SAR.Acknowledgements
This research was partly supported by Siemens Medical Solutions USA and by NIH R21 EB022309.References
[1] Meyer CH, Hu B, Nishimura DG, Macovski A. Fast spiral coronary artery imaging. Magn Reson Med. 1992;28:202-213.
[2] Li Z, Karis JP, Pipe JG. A 2D spiral turbo-spin-echo technique. Magn Reson Med. 2018;80:1989–1996.
[3] Block W, Pauly J, Nishimura D. RARE spiral T2-weighted imaging. Magn Reson Med. 1997;37:582–590
[4]. Hennig J, Menza M, Barghoorn A, Riemenschneider B, Kroboth S, Zaitsev M. Spiral RARE with annular segmentation. Proceedings 27th Annual Meeting ISMRM, Montreal. 2019:4632.
[5] Johnson B, Fram EK, Drayer BP, et al. Evaluation of shared-view acquisition using repeated echoes (SHARE): a dual-echo fast spin-echo MR technique. Am J Neuoradiol 1994;15:667-673.
[6] Meyer CH, Pauly JM, Macovski A, Nishimura DG. Simultaneous spatial and spectral selective excitation. Magn Reson Med. 1990;15:287–304.
[7] Tan H, Meyer CH. Estimation of k-space trajectories in spiral MRI. Magn Reson Med 2009;61:1396–1404.
[8] Chen W, Sica CT, Meyer CH. Fast conjugate phase image reconstruction based on a Chebyshev approximation to correct for B0 field inhomogeneity and concomitant gradients. Magn Reson Med. 2008. 60(5): 1103-1111.
Figures
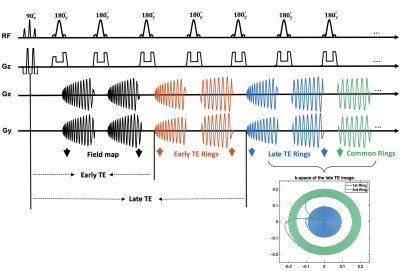
Figure 1. Spiral ring pulse sequence diagram. Spectral-spatial RF water excitation 90° pulses were used at the beginning to suppress lipids. In a multi-shot acquisition, the first interleaved, black colored spiral arms were used for field map acquisitions, with a 1-ms echo shift in the next shot. The center of k-space was sampled both at an early echo time and a late echo time by the first and second spiral rings, respectively, while the outer k-space regions were sampled by the same outer spiral rings. These green common echoes were then shared by both the orange and blue echoes.