3709
PASTeUR package extension with MP2RAGE for robust T1 mapping technique in parallel transmit at 7T
Franck Mauconduit1, Aurélien Massire2, Vincent Gras1, Alexis Amadon1, Alexandre Vignaud1, and Nicolas Boulant1
1DRF/Joliot/Neurospin & Université Paris-Saclay, CEA, Gif-sur-Yvette, France, 2Siemens Healthcare SAS, Saint Denis, France
1DRF/Joliot/Neurospin & Université Paris-Saclay, CEA, Gif-sur-Yvette, France, 2Siemens Healthcare SAS, Saint Denis, France
Synopsis
Universal Pulses (UP) were recently proposed as a plug-and-play pTx solution. To extend the so-called PASTeUR package of anatomical sequences with a mapping technique, we focused on a MP2RAGE acquisition that has shown to be robust against receive profiles when acquired at 7T in single channel transmission. UP provide the advantage of intrinsically correcting for flip angle variations throughout the brain, leading to an increased SNR in regions usually left with low B1+ intensity. Moreover, T1 quantification can be obtained without the need for B1+ acquisition and additional post-processing.
Introduction
Parallel transmission (pTx) seems to be the most promising solution to tackle the B1+ inhomogeneity encountered at ultra-high field MRI (≥7T). However, this solution comes with a number of obstacles for routine due to the need of subject-based calibrations (field map acquisition), data processing and online pulse design. Universal Pulses (UP) were recently proposed as a plug-and-play solution [1] with no need for subject-specific calibration. A set of standard non-selective 3D acquisitions were developed in a so-called PASTeUR [2] package including 3D GRE, MPRAGE, SPACE with FLAIR and DIR preparations. To extend this set of weighted images with a mapping technique, we focused on a MP2RAGE acquisition that has shown to be robust against receive profiles when acquired at 7T in single channel transmission [3,4]. By combining the two volumes acquired at two different inversion times, a uniform T1-weigthed volume (UNI) as well as a T1 map can be derived easily. In this study, we analyze the results obtained with MP2RAGE using UP and compare with a pseudo CP mode pTx acquisition that mimics the results in combined standard 7T MRI mode.Methods
Acquisitions were performed on a healthy volunteer on an investigational Magnetom 7T scanner (Siemens Healthineers, Erlangen, Germany) equipped with a 8Tx/32Rx pTx coil (Nova Medical, Wilmington, MA, USA). RF transmit field maps were measured using a magnetization prepared turbo-FLASH [5] with 5mm isotropic resolution. For MP2RAGE acquisition in CP mode, the inversion pulse was an adiabatic hyperbolic secant pulse with a maximum available voltage of 170V in pTx. The excitation pulse was set using a reference voltage of 65V chosen to give the target flip angle in the central region of the brain with 10% overshoot, as it can be the case in single channel mode. In UP mode, the sequence incorporated non-selective kT-point pulses [6] designed on a database of 20 subject B1+ field maps [7] and more specifically a 3.68 ms-inversion pulse, designed with a GPU-based Bloch simulator, and a small tip angle excitation pulse lasting 570 µs. The whole setup was performed to be compatible with Siemens protected mode step 2.3, i.e. with peak amplitude limits of 165 V and average power limits of 1.5 W per channel and 8 W total for the coil of interest. In both cases, MP2RAGE acquisition was 0.8mm isotropic with a TR of 6000ms, TIs of 800ms/2700ms, an acceleration factor of 3 in the phase-encoding direction with GRAPPA reconstruction, for a total acquisition time of 11 min 36 s. UNI volumes and T1-maps were reconstructed using an in-house Matlab algorithm based on [3,4]. A brain mask was extracted using bet (FSL, FMRIB Software) on the second inversion volume in UP mode and applied both on CP and UP T1-maps to reconstruct histogram of the T1 values.Results
Figure 1 highlights the important signal heterogeneity of the MP2RAGE native volumes obtained in CP mode as compared with UP mode. Figures 2 and 3 report orthogonal views of UNI volumes and T1-maps respectively. The overall image quality is relatively similar when comparing CP versus UP mode, noticing a significant signal-to-noise ratio (SNR) improvement in UPs throughout the brain and even more present in the cerebellum. Figure 4 shows histograms of T1 values obtained throughout the brain using an extraction mask. An overall 3.3% difference between the two main modes is observed with a global shift toward higher values in CP mode. T1 mean and standard deviation values measured within white matter ROIs depicted in figure 3 are summarized in table 1. Locally, ROI values show a 7% increase in white matter T1 between CP and UP mode. Moreover, T1 standard deviation confirms that CP mode results in a lower SNR than UP mode.Discussion
Despite the signal drop observed in the cerebellum in native inversion volumes of the CP mode, the reconstructed UNI volume largely recovers the heterogeneity due to reception field bias cancellation. However, the brain extraction using bet (FSL, FMRIB Software) did not perform as well on CP mode as compared to the homogeneous UP acquisition. Therefore, the mask obtained on UP volume was applied on both T1 maps. Although acquiring a B1+ map is a possible strategy to improve T1 quantification when acquiring in CP mode [8,9], it requires extra acquisition time and additional post-processing step. Incorporating UP design in the MP2RAGE sequence provides the advantage of intrinsically correcting for flip angle variations throughout the brain, leading to an increased SNR in regions usually left with low B1+ intensity. This work thereby shows that quantitative MRI methods can also benefit from UPs to increase SNR, accuracy and potentially spare the user additional scans [9].Conclusion
This work reports the development and validation of a MP2RAGE acquisition embedding a calibration-free pTx set of pulses to perform robust T1 mapping in complement with existing anatomical sequences using universal pulses. Following these results, the next PASTeUR version should soon incorporate MP2RAGE acquisition and inline reconstruction of UNI volume and T1 maps.Acknowledgements
This work has been supported by the Leducq Foundation large equipment ERPT program.References
[1] Gras et al., MRM 77, 2017. [2] Gras et al., ISMRM 2019, p4626. [3] Marques et al., NeuroImage 49, 2010. [4] Marques and Gruetter, PLoS One 8, 2013. [5] Amadon et al., ISMRM (2012), p3358. [6] Cloos et al., MRM 80, 2012. [7] Gras et al., PlosOne 12, 2017. [8] Massire et al., NeuroImage 143, 2016. [9] Haast et al., HBM 39, 2018. [10] Leroi et al., ISMRM (2019), p315.Figures
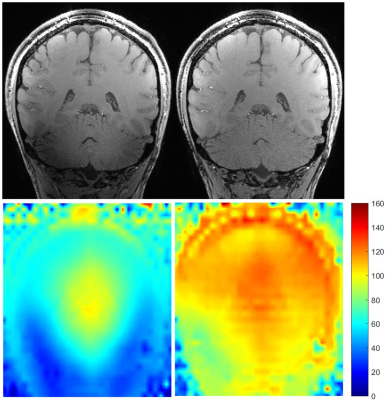
Figure 1 - Upper
images are coronal views of proton density contrast (second inversion of
MP2RAGE acquisition) in CP mode (upper left) and UP mode (upper right). Lower
left image is a relative B1+ map acquired in CP mode. Lower right is a Bloch
simulation relative B1+ map of the MP2RAGE excitation pulse in UP mode using
the 8-channels transmit field maps. Affected by the very low B1+ field, the
cerebellum exhibits a heterogeneous signal in CP mode whereas universal
kT-points maintain a uniform excitation throughout the brain in UP mode.
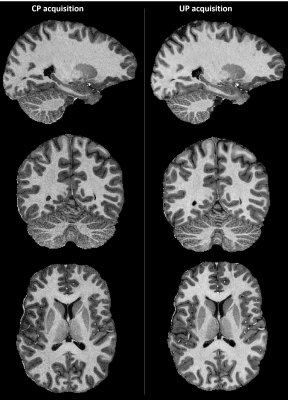
Figure 2 - Orthogonal views of UNI contrast computed from the two inversion volumes
of the MP2RAGE acquisition in CP mode (left) and UP mode (right). Image
contrast is very similar throughout the brain with a slightly decreased quality
(noisier and less detailed) in the cerebellum in CP mode where B1+ is particularly
low as shown in figure 1.
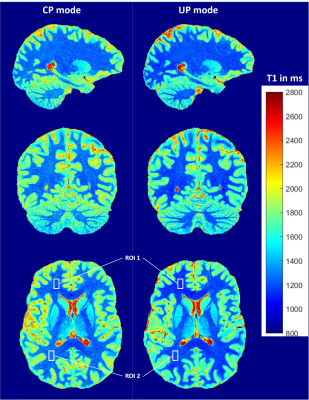
Figure 3 - Orthogonal views of T1 maps obtained for both CP mode (left) and UP mode
(right). T1 maps were computed by injecting UNI signal intensities and sequence
parameters in Bloch equations simulation.
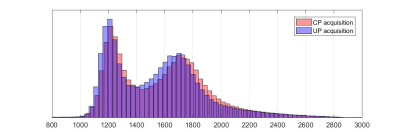
Figure 4 - Histogram plots of the T1 values extracted from the brain for both CP
mode and UP mode. A brain mask was computed using bet on the second MP2RAGE
volume and applied to the T1 maps.
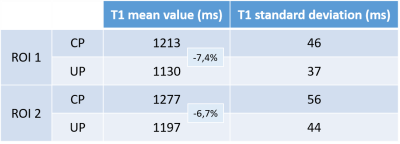
Table 1 - Mean T1 values and standard deviations for CP and UP mode extracted from
two ROIs displayed in the axial view of figure 3.