3690
Mapping oxygen extraction fraction using a tailored parallel transmission RF pulse at 7 T1Wellcome Centre for Integrative Neuroimaging, FMRIB Division, NDCN, University of Oxford, Oxford, United Kingdom
Synopsis
T2‐relaxation under‐spin‐tagging (TRUST) is a robust spin tagging based method to quantify oxygen extraction fraction (OEF), but it lacks spatial specificity. Recently O’Brien et al. proposed a method involving multiple saturation pulses to achieve spatial specificity. Parallel transmission (pTx) provides additional degrees of freedom for spatial localisation. A pTx RF pulse design strategy based on a shells trajectory is applied to perform regional OEF measurement at 7 T. Initial in-vivo results acquired from one healthy subject showed that spatial localisation of OEF could be achieved.
Introduction
Oxygen extraction fraction (OEF) is an important parameter for the understanding of normal and abnormal physiology in the brain. Lu and Ge1 introduced an MR-based method named T2‐relaxation-under‐spin‐tagging (TRUST) to measure OEF by labelling spins in the venous blood and performing pair-wise subtraction in the sagittal sinus. OEF is then inferred based on the known relationship between blood T2 and blood oxygenation levels1. Although proven to be a robust non-invasive technique to measure cerebral OEF, TRUST has one key limitation: it does not provide spatial specificity. O’Brien et al. applied a train of water suppression enhanced through T1 effects (WET) saturation pulses to measure regional OEF using selective localised-TRUST (SL-TRUST) at 3 T2. Parallel transmission (pTx) allows different RF waveforms to be played out in different transmit channels, thus providing more degrees of freedom for spatial selectivity. In this work, we present a single pTx pulse design strategy to measure regional OEF in the brain at 7 T.Methods
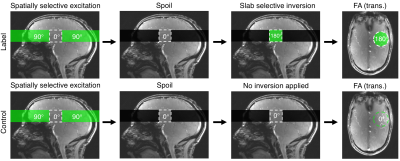
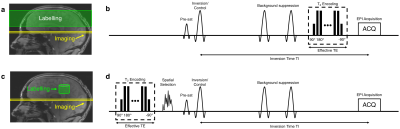
B1+ and B0 maps were acquired from a single subject (25 years, male) on a Siemens (Erlangen, Germany) Magnetom 7 T scanner equipped with an 8 channel pTx system, and a Nova Medical Inc. (Wilmington, MA, USA) 8Tx/32Rx head coil. B1+ maps were acquired using a 2D “STE first” phase-cycled DREAM sequence3,4. B0 maps were acquired using a dual-echo gradient-echo sequence. A brain mask was generated from the raw images in the B0 mapping sequence using BET5.The labelling strategy consists of the following steps. First, a spatially selective pTx pulse excites a 3D shape that excludes the region-of-interest (ROI), which is then saturated by a spoiler gradient. Second, a slab-selective inversion pulse is played out to invert the remaining longitudinal magnetisation in the ROI. In the control case, the same spatially selective pulse is used, but without a subsequent inversion pulse, therefore creating a signal difference solely from the venous blood in the ROI (see Fig. 1 and Fig. 2). The T2 encoding is moved to the front of the sequence, unlike the conventional TRUST where it is at the end.
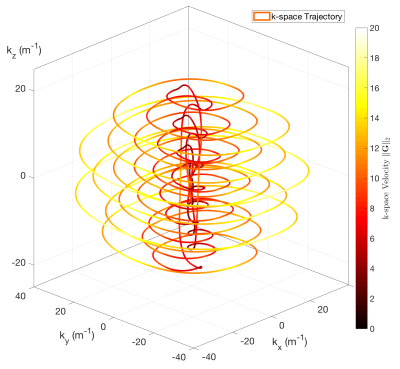
A shells trajectory proposed by Davids et al. was used for the pulse design6 (see Fig. 3). No gradient trajectory optimisation was performed in this study. A cylindrical ROI with a height of 40 mm was chose. The pulse design employed the variable-exchange algorithm, which was based on a method proposed by Dupas et al.7. The regularisation constant λ values were chosen heuristically to produce acceptable results in FA with a feasible RF voltage. The optimisation algorithm mentioned above was implemented in MATLAB (The MathWorks, Natick, MA, USA)8. The designed RF pulse was then incorporated into the TRUST sequence to evaluate its performance. T2 encoding, pre-saturation and double inversion pulses were turned off to isolate the effects proposed RF pulse. The inversion time was set to lowest possible value of 73ms to minimise relaxation effects. Five slices in the were acquired using an EPI readout.
Results
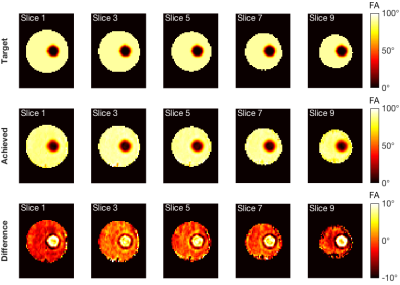
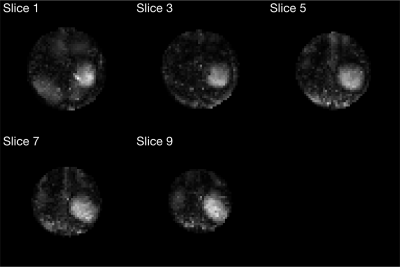
Simulation results were shown in Fig 4. A root mean square error (RMSE) of 6.71% was achieved. Larger errors occurred in the ROI compared to the rest of the slices. Raw EPI images were masked and shown in Fig 5. A circular pattern could be observed in each slice, but the boundary of such a pattern varied across slices. Moreover, saturation of signal outside of the ROI was not achieved with great accuracy. The sum of pixel values in the ROI accounted for an average of 27.1% of the sum of pixel values in the whole masked brain slice.Discussions
Higher intrinsic SNR at 7T is beneficial to the TRUST method, enabling OEF measurements of smaller regions. However, spatial selection based on multiple WET saturation pulses might not be suitable for 7T, as the saturation performance may be impaired due to B1+ inhomogeneity. In this abstract, we introduced a single tailored pTx RF pulse design to achieve spatial localisation in the presence of B1+ inhomogeneity.Although the magnetisation was not controlled beyond the slab of interest, OEF measurements should not be affected since the spatially selective pulse was applied in both the label and control conditions, meaning any errors should subtract. Simulation results showed that spatial localisation could be achieved with 6.71% RMSE error. However, in-vivo results showed that although the ROI was clearly identified, saturation of signal outside of the ROI was not achieved with great accuracy. Imperfect timing synchronisation between the RFPA and gradient system or inaccurate playout of the prescribed complex gradient waveforms might explain the pulse performance. Calibrating these imperfections has been shown to improve the accuracy of pTx pulses9.
Conclusion
A pTx RF pulse design is presented for regional OEF measurement at 7 T. Initial in-vivo results showed the feasibility of the method, but RF/gradient system imperfections need to be corrected for complete saturation of tissue outside the selected volume.Acknowledgements
The authors acknowledge the China Oxford Scholarship Fund, Royal Academy of Engineering, Dunhill Medical Trust, University College Oxford and the NIHR Oxford Biomedical Research Centre. The Wellcome Centre for Integrative Neuroimaging is supported by the Wellcome Trust (203139/Z/16/Z). The support of the UK Medical Research Council UK7T Network is acknowledged (MR/N008537/1). The support of support from the Engineering and Physical Sciences Research Council (EPSRC) and Medical Research Council (MRC) Centre for Doctoral Training in Biomedical Imaging (grant EP/L016052/1) is also acknowledged.References
1. Lu, H. and Y. Ge, Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med, 2008. 60(2): p. 357-63.
2. O'Brien, C., et al., Volume-localized measurement of oxygen extraction fraction in the brain using MRI. Magn Reson Med, 2019. 82(4): p. 1412-1423.
3. Nehrke, K. and P. Bornert, DREAM--a novel approach for robust, ultrafast, multislice B(1) mapping. Magn Reson Med, 2012. 68(5): p. 1517-26.
4. Tse, D.H., et al., Encoding methods for B1(+) mapping in parallel transmit systems at ultra high field. J Magn Reson, 2014. 245: p. 125-32.
5. Smith, S.M., Fast robust automated brain extraction. Hum Brain Mapp, 2002. 17(3): p. 143-55.
6. Davids, M., et al., Fast three-dimensional inner volume excitations using parallel transmission and optimized k-space trajectories. Magn Reson Med, 2016. 76(4): p. 1170-82.
7. Dupas, L., et al., Two-spoke placement optimization under explicit specific absorption rate and power constraints in parallel transmission at ultra-high field. J Magn Reson, 2015. 255: p. 59-67.
8. Constrained Nonlinear Optimization Algorithms. [cited 2017; Available from: https://uk.mathworks.com/help/optim/ug/constrained-nonlinear-optimization-algorithms.html#brnox01.] 9. Cavusoglu, M., et al., Correction of parallel transmission using concurrent RF and gradient field monitoring. MAGMA, 2017. 30(5): p. 473-488.
Figures