3686
Partial Fourier Readout in Readout Segmentation of Long Variable Echo Trains-Based Imaging In Nasopharyngeal Carcinoma1Radiology, Hubei Cancer Hospital, Tongji Medical College, Huazhong University of Science and Technology, wuhan, China, 2MR Scientific Marketing, Siemens Healthcare, Wuhan, China, 3MR Application Predevelopment,Siemens Healthcare, Erlangen, Germany
Synopsis
There is a potential influence of partial Fourier readout on readout segmentation of long variable echo trains (RESOLVE)-based intravoxel incoherent motion (IVIM) and diffusion kurtosis imaging (DKI) models. The aim of this study was to investigate the influence of partial Fourier readout on RESOLVE-based IVIM and DKI parameters in nasopharyngeal carcinoma. The results showed that partial Fourier readout has limited influence on DKI parameters, but significant influence on the perfusion parameters of IVIM.
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common malignancies in Chinese and Southeast Asians. Although the intravoxel incoherent motion (IVIM) and diffusion kurtosis imaging (DKI) methods based on conventional single-shot echo-planar imaging (ss-EPI) have been used in tumor detection, staging, characterization, and treatment response prediction for patients with NPC 1-5, the diffusion images are usually affected by significant susceptibility artifacts in regions near bones and air. Readout segmentation of long variable echo trains diffusion-weighted imaging (RESOLVE DWI), which has higher spatial resolution and fewer susceptibility artifacts, has shown potential to solve this problem 6; however, it requires longer acquisition time, especially for IVIM and DKI imaging. Previous studies have shown that partial Fourier readout could be used to reduce acquisition times for RESOLVE DWI; however, it affected the apparent diffusion coefficient (ADC) values 7. We are not aware of any studies that have investigated the effect on IVIM- and DKI-derived parameters based on RESOLVE DWI. The purpose of this study was to evaluate the effect of partial Fourier readout for RESOLVE-based IVIM- and DKI-derived parameters for patients with NPC.Methods
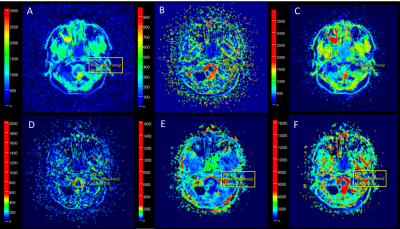
Twelve pathologically confirmed NPC patients who underwent conventional imaging and multi-b-value DWI before receiving therapy were enrolled in this study. All patients were imaged using a 3T MRI scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) with a 20-channel head-and-neck array coil. Two groups of axial DWI data with 11 b-values were obtained using the RESOLVE sequence with (5/8) and without partial Fourier readout (TR/TE1/TE2, 3960/82/98 ms; section thickness/intersection gap, 4/0.4 mm; matrix size = 134 × 134; field-of-view (FOV) = 258 × 258 mm2; b-value = 0/10/20/40/80/150/200/400/800/1500/2000 s/mm2, readout segments, 5; and scan time, 8:59 min and 14:51 min, respectively). IVIM and DKI model fitting were conducted using the prototype software MR Body Diffusion Toolbox V1.3 (Siemens Healthcare, Erlangen, Germany). Referencing the corresponding conventional images, three regions of interest (ROIs) (larger than 20 pixels and avoiding areas of necrosis) were determined and manually drawn on the b = 800 s/mm2 (without partial Fourier readout) images in the slice containing the largest section of the tumor and its adjacent up/down sections by a radiologist with more than 10 years of experience, and then confirmed by a second radiologist with more than 15 years of experience. Nine b-values from 0 to 800 s/mm2 were used to calculate IVIM-derived parameters (ADC, perfusion fraction (fp), water diffusion coefficient (D), pseudodiffusion coefficient (Dp); Figures 1 A-D, respectively). Five b-values with 200, 400, 800, 1500, 2000 s/mm2 were used to calculate DKI-derived parameters (mean diffusion coefficient (MD), mean kurtosis (MK); Figures 1 E-F, respectively). All parameter values were averaged. Bland-Altman analyses were used to evaluate the differences in IVIM- and DKI-derived parameters between those with and without partial Fourier readout. The mean ± 1.96 times standard deviation (SD) was defined as the limits of agreement (LOA). Statistical analyses were performed using GraphPad Prism software (v5.0, La JoIIa, USA).Results
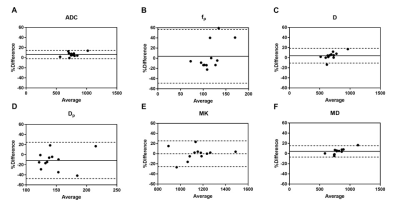
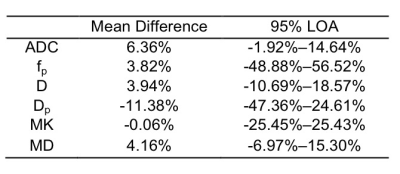
Figure 1 provides images from a patient with NPC derived with IVIM and DKI. Figure 2 and Table 1 show the results of Bland-Altman analyses and the LOA of percentage differences between IVIM- and DKI-derived parameters with and without partial Fourier readout. Overall, IVIM-derived parameters demonstrated a relatively larger difference than those derived with DKI. As shown in Figure 2A, the mean difference for ADC was 6.36%; the LOA was -1.92%–14.64%. Figures 2B and 2C show 3.82% and 3.94% mean differences for fp and D, respectively; the LOAs were -48.88%–56.52% and -10.69%–18.57%, respectively. One outlier can also be observed at 59.39% in Figure 2B. The highest difference of -11.38% can be observed for Dp, with the LOA at -47.36%–24.61%, as shown in Figure 2D. MK shows the smallest difference of -0.06%; the LOA was -25.45%–25.43%, as shown in Figure 2E. Figure 2F shows a 4.16% mean difference for MD; the LOA was -6.97%–15.30%.Discussion
RESOLVE is a technique that divides k-space in the readout direction and collects signals by multi-shot 6. Using partial Fourier readout to collect the signal of some of the divided segments shortens the imaging time. Even though partial Fourier readout saved nearly 40% of acquisition time in this study, it had significant effects on IVIM-derived perfusion parameters fp and Dp (in some cases showing a 40%–60% difference), but limited effects on DKI-derived parameters (<26%). This may be because the fitting of IVIM relies primarily on multiple small b-values, which are more affected by image quality. Ariya et al 7demonstrated that the signal intensity and signal-to-noise ratio (SNR) of an object decreased, while the noise and artifacts increased as the ratio of partial Fourier readout decreased. This is consistent with the large differences we observed for IVIM-derived parameters.Conclusion
Partial Fourier readout can significantly shorten the acquisition time of RESOLVE-based IVIM and DKI imaging. This study indicates that while it may have limited influence on DKI parameters, partial Fourier readout appears to have significant influence on the perfusion parameters of IVIM.Acknowledgements
NoneReferences
[1] Xiao Y, Pan J, Chen Y, Chen Y, He Z, Zheng X. Intravoxel incoherent motion-magnetic resonance imaging as an early predictor of treatment response to neoadjuvant chemotherapy in locoregionally advanced nasopharyngeal carcinoma. Medicine 2015;94.
[2] Lai V, Li X, Lee VHF, Lam KO, Fong DYT, Huang B, et al. Nasopharyngeal carcinoma: comparison of diffusion and perfusion characteristics between different tumour stages using intravoxel incoherent motion MR imaging. Eur Radiol 2014;24:176–83.
[3] Lai V, Li X, Lee VHF, Lam KO, Chan Q, Khong P-L. Intravoxel incoherent motion MR imaging: comparison of diffusion and perfusion characteristics between nasopharyngeal carcinoma and post-chemoradiation fibrosis. Eur Radiol 2013;23:2793–801.
[4] Guo W, Luo D, Lin M, Wu B, Li L, Zhao Y, et al. Pretreatment intra-voxel incoherent motion diffusion-weighted imaging (IVIM-DWI) in predicting induction chemotherapy response in locally advanced hypopharyngeal carcinoma. Medicine 2016;95.
[5] Huang W-Y, Li M-M, Lin S-M, Chen F, Yang K, Zhu X-L, et al. In Vivo Imaging Markers for Prediction of Radiotherapy Response in Patients with Nasopharyngeal Carcinoma: RESOLVE DWI versus DKI. Scientific Reports 2018;8:15861.
[6] Porter DA, Heidemann RM. High resolution diffusion‐weighted imaging using readout‐segmented echo‐planar imaging, parallel imaging and a two‐dimensional navigator‐based reacquisition. Magnetic Resonance in Medicine: an Official Journal of the International Society for Magnetic Resonance in Medicine 2009;62:468–75.
[7] Ariya W, Suzuki R, Hasegawa T. The Influence of the Decrease in Data Acquisition Ratio Resulted from Readout Partial Fourier during Readout Segmented EPI on ADC Map: A Phantom Study. Nihon Hoshasen Gijutsu Gakkai Zasshi 2019;75:133–42.
Figures