3679
Fast, Automated DTI-based Thalamus Nuclei Segmentation1Department of Electrical and Computer Engineering, University of Arizona, Tucson, AZ, United States, 2College of Medicine, University of Arizona, Tucson, AZ, United States, 3Department of Biomedical Engineering, University of Arizona, Tucson, AZ, United States, 4Department of Surgery, University of Arizona, Tucson, AZ, United States, 5Department of Medical Imaging, University of Arizona, Tucson, AZ, United States
Synopsis
Fast, accurate, and automatic thalamus segmentation is critical in evaluating the roles of individual thalamic nuclei in pathology and treatment. Many segmentation techniques developed to date involve the use of Diffusion Tensor MRI and are inhibited by their reliance upon i) time-consuming processing to produce an initial mask for the entire thalamus, and ii) orientation distribution functions incapable of modeling intricate small-scale fiber tract geometries. We present a technique that addresses these issues by i) greatly accelerating the masking process via template-based registration, and ii) using constrained spherical deconvolution to produce enhanced ODFs that drive a modified k-means clustering algorithm.
Introduction
The thalamus is a bilateral deep brain structure consisting of histologically and functionally distinct nuclei which are increasingly implicated for their roles in pathology and treatment. Thalamic nuclei show selective vulnerability in schizophrenia1,2 and Alzheimer’s Disease3, and are targeted in deep brain stimulation for treatment of essential tremor4. Individual nuclei are largely invisible in conventional T1 and T2 weighted MRI and yet there is a critical need for fast and accurate methods for thalamic nuclei parcellation. Battistella et al5 have proposed a DTI-based technique using k-means clustering of orientation distribution function (ODF) coefficients to segment the nuclei. This method is robust and replicable, but limited in two respects: i) it relies on time consuming cortical parcellation to produce a thalamic mask, and ii) it relies on Q-Ball Imaging (QBI), which cannot account for partial volume influences or model complex white matter (WM) microstructure such as intravoxel fiber crossing when generating ODFs. In this work, we address these two limitations by: i) using template-based registration to accelerate masking, and ii) using either Single Shell (SS) or Multi-shell, Multi-tissue (MSMT) Constrained Spherical Deconvolution (CSD)6,7 to compute ODFs. We implemented and evaluated the masking and ODF techniques via individual parcellations performed on 30 subjects.Methods
Thalamus masking: Masking was performed on the left and right halves of the thalamus for all subjects. The thalamus mask from a template image (generated via the co-registration and averaging of 20 WMn MPRAGE volumes with manually segmented thalami8 ) was transferred to the individual T1-weighted space via nonlinear registration in ANTs9. Masks were warped to the individual DWI space and subsequently refined via the process used in [5]: fractional anisotropy (FA) and tissue probability maps were used to eliminate voxels within the masks exhibiting FA values above 0.55 or cerebrospinal fluid (CSF) tissue probability above 0.05. Figure 1 gives an overview of the masking procedure. For the purposes of comparison, masks were also generated via Freesurfer cortical parcellation as outlined in [5].ODF generation and segmentation: Both single-shell and multi-shell multi-tissue CSD can model more complex small-scale tract geometries than QBI, while MSMT CSD is also able to independently model ODFs of distinct tissues (WM, CSF etc.) present within a voxel. Order six ODFs (corresponding to a 28 coefficient spherical harmonic expansion) were calculated at each DTI voxel via three algorithms: i) QBI, ii) SS CSD (using a SS DTI collection synthesized from the b=0 and 3000 s/mm2 shells), and iii) white matter-only MSMT CSD (using all collected shells). Figure 2 highlights differences between ODFs. Segmentation was performed by employing k-means clustering (n=7 clusters) with a mixed L2 norm scaled to balance voxel proximity with ODF similarity:
$$ D = \alpha d_{vox} + (1-\alpha)\Gamma d_{ODF}$$
Data collection: After informed consent, 15 individuals were scanned twice, yielding 30 datasets in total. Each dataset consists of two types of images: T1-weighted Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) (TR/TE=2530/3.3ms, TI=1000ms, 1mm3 resolution), and DTI (EPI with TR/TE = 3700/115ms, 2mm3 resolution, 130 diffusion directions: 14, 20, 32, and 64 directions at b-values of 0, 1000, 2000, and 3000 s/mm2, respectively).
Analysis: To evaluate each parcellation method, the T1 MPRAGE was segmented using the template atlas method described in [8]. Briefly, after nonlinear registration of the T1 MPRAGE to the template, thalamic nuclei from the template space were warped to the individual space. Dice scores were computed between these nuclei and the clusters corresponding to each of the three ODF generation methods:
$$Dice(L_{nucleus},L_{cluster}) = 2\frac{||L_{nucleus} \cap L_{cluster}||}{||L_{nucleus}|| + ||L_{cluster}||}$$
Dice scores were also computed between the Freesurfer and template-based masks.
Results
The Freesurfer-based masking required an average of 485.7 +/- 62.4 minutes, while the template-based registration masking required 15.5 +/- 2.2 minutes. Average Dice scores between the Freesurfer-based and template-based masks were 0.87 +/- 0.02 and 0.88 +/- 0.02 for the right and left halves, respectively. Individual left thalamus segmentations using QBI, SS CSD, and MSMT CSD for two individuals are shown in figure 3. Figure 4 gives mean +/- standard deviation of Dice between each of the three parcellation methods and the template nuclei warped to individual space. At a p-value of 0.05, the paired sample t-test showed statistically significant differences between QBI and SS CSD in seven of the 11 left and right nuclei; MSMT CSD was significantly higher in eight left and six right nuclei. QBI tested significantly higher (than either method) in the Mediodorsal nucleus only. No differences between SS CSD and MSMT CSD Dice scores tested as significant.Discussion
The average Dice scores between the two masking techniques demonstrate that the template-based technique generates highly similar masks compared to Freesurfer at a significant computational savings, while being ~32X faster. Evaluation of individual parcellations and Dice scores indicate that all techniques capture larger nuclei more successfully.Conclusion
We have demonstrated an improved scheme for DTI-based thalamus parcellation. By using a template-based registration masking technique, we have significantly reduced processing while maintaining strong correspondence with the previously used masks. Using SS CSD for ODF generation showed a statistically significant advantage over QBI in a majority of the thalamic nuclei. MSMT CSD also shows performance gains over QBI, but not over SS CSD.Acknowledgements
No acknowledgement found.References
1. Andreasen, Nancy C. 1997. “The Role of the Thalamus in Schizophrenia.” The Canadian Journal of Psychiatry 42 (1): 27–33. https://doi.org/10.1177/070674379704200104.
2. Byne, W., M. S. Buchsbaum, E. Kemether, E. A. Hazlett, A. Shinwari, V. Mitropoulou, and L. J. Siever. 2001. “Magnetic Resonance Imaging of the Thalamic Mediodorsal Nucleus and Pulvinar in Schizophrenia and Schizotypal Personality Disorder.” Archives of General Psychiatry 58 (2): 133–40.
3. Braak, H., and E. Braak. 1991. “Alzheimer’s Disease Affects Limbic Nuclei of the Thalamus.” Acta Neuropathologica 81 (3): 261–68.
4. Benabid, A. L., P. Pollak, D. Hoffmann, C. Gervason, M. Hommel, J. E. Perret, J. de Rougemont, and D. M. Gao. 1991. “Long-Term Suppression of Tremor by Chronic Stimulation of the Ventral Intermediate Thalamic Nucleus.” The Lancet, Originally published as Volume 1, Issue 8738, 337 (8738): 403–6. https://doi.org/10.1016/0140-6736(91)91175-T.
5. Battistella, Giovanni, Elena Najdenovska, Philippe Maeder, Naghmeh Ghazaleh, Alessandro Daducci, Jean-Philippe Thiran, Sébastien Jacquemont, et al. 2017. “Robust Thalamic Nuclei Segmentation Method Based on Local Diffusion Magnetic Resonance Properties.” Brain Structure and Function 222 (5): 2203–16. https://doi.org/10.1007/s00429-016-1336-4.
6. J.-D. Tournier, F. Calamante, and A. Connelly. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage, 35 (2007), pp. 1459–72.
7. B. Jeurissen, J.-D. Tournier, T. Dhollander, A. Connelly, and J. Sijbers. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. NeuroImage, 103 (2014), pp. 411–426.
8. Saranathan et al. An anatomical atlas for segmentation of thalamic nuclei from conventional 3T MRI. Proceedings of the 27th ISMRM, Montreal, Canada. p2640 (2019)
9. “ANTs by Stnava.” n.d. Accessed March 31, 2019. http://stnava.github.io/ANTs/.
Figures
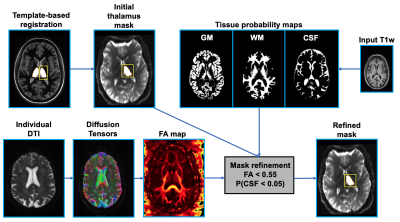
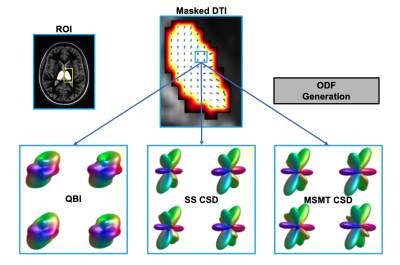
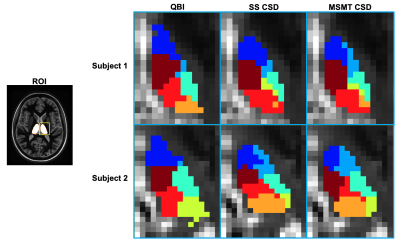
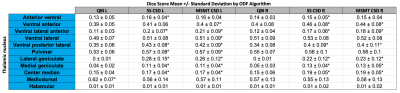
Figure 4: Mean Dice scores +/- standard deviation for 11 thalamic nuclei versus parcellation clusters using QBI, SS CSD, and MSMT CSD ODFs. Paired t-tests were run between all methods for n=30 subjects.
* denotes statistically significant difference between QBI/SS CSD/MSMT CSD at p=0.05 level of significance.