3648
An MR guided focused ultrasound software with Gadgetron reconstruction1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
Synopsis
An integrated focused ultrasound guidance software named MARFit was developed base on the framework of Gadgetron. The software realized automatically focus localization and real-time temperature change monitoring during HIFU therapy. These features has been evaluated in animal experiments. The software makes the post processing procedure easily translated between different vendors, and could be a powerful tool for MR guided focused ultrasound therapy.
Introduction
Focused ultrasound is a promising non-invasive treatment for various brain diseases. However, its biggest challenge is to precisely localize the focus. MR-ARFI allowed to targeting the focus through micro-scale local displacement. This procedure could be iterative, which raises the need of MR-ARFI information feedback and an easy-to-use tool for guidance. Besides, during the treatment, the temperature change should always be monitored for treatment evaluation or safety concerns in a real-time way. In this study, we developed an MR guided software named as MARFit. The software could be used to automated localize the transcranial ultrasound focus at the pre-treatment planning stage, and monitoring the temperature change in real-time during treatment. The software make the post processing procedure easily translated between different vendors, and could be a powerful tool for MR guided focused ultrasound therapy.Materials and methods
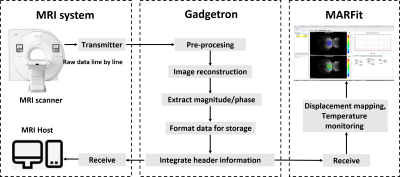
The MARFit software was developed based on Visualization ToolKit (VTK). The MR image reconstruction was implemented in an open source platform Gadgetron. MR-ARFI sequence was adapted to transmitting the k-space data line by line to an installed Gadgetron server through a local area network. The integrated header information including the imaging orientation and position were fed back to the MR host and into the MARFit software. The information of the displacement encoding gradient (Amplitude G and duration $$$\tau$$$ of displacement encoding gradient) and temperature mapping (B0 and TE) were also extracted and transmitted to MARFit, where the displacement and temperature map were calculated and visualized (Figure 1).For focus localization, four images of MR-ARFI1 were obtained. 2 images ($$$\phi^+$$$ and $$$\phi^-$$$) with opposite polarity of DEG were acquired during ultrasound pulse ON to increase the detection sensitivity. Two images ($$$\phi_{ref}^+$$$ and $$$\phi_{ref}^-$$$) identical to the first 2 images but without HIFU pulse were acquired to avoid the eddy current problem raised from inverted gradient polarity. The k-space data of these 4 images were acquire interleaved to reduce the ultrasound duty cycle. Then the displacement was calculated by: $$D=\frac{(\phi^+-\phi^-)-(\phi_{ref}^+-\phi_{ref}^-)}{2\gamma G\tau}$$
, where $$$\gamma$$$ is the gyro-magnetic ratio. Based on the displacement map, the focus of the ultrasound would be automatically localized through searching the maximum displacement within the selected ROI. The localization information was then transformed to the MRI physical coordinate according to the imaging orientation and position information.
For temperature monitoring, the temperature change at time t against the selected reference(s) ($$$\phi_{ref}$$$) can be expressed as2: $$\triangle T=\frac{\phi-\phi_{ref}}{\gamma\alpha B_0TE}$$
, where $$$\alpha $$$ is the temperature sensitivity of PRF. The temperature map and temperature change curve of an arbitrary pixel would be generated using a multiple reference method to mitigate the motion induced calculation error. Through a close loop feedback based on model predictive control (MPC) algorithm, the temperature can be controlled at a target value. The thermal dose (equivalent minutes at 43ºC) information could be also produced using the temperature images after the acquired images were registered to the reference images.
The transcranial focus localization function of the software were tested in a 3.0T system (uMR 790, United Imaging Healthcare, Shanghai, China) in monkeys with IRB approval, while the temperature monitoring and control function were tested in rabbit thigh due to the safety concern.
Results
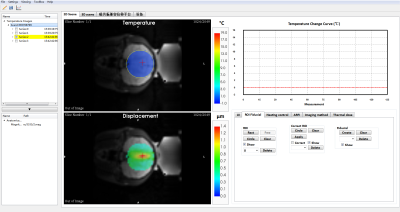
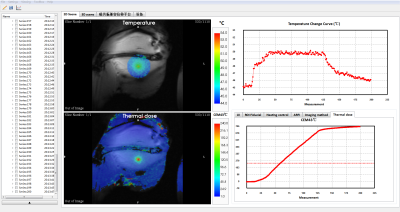
The reconstruction of ARFI and thermometry rawdata was finished and the image data were transmitted from the Gagdetron server to MARFit without delay. Figure 2 illustrated the transcranial focus localization result. The maximum displacement was 1.36 μm. The total acquisition time was 4 min 16 s. The focus was highlighted by a cross arrow.Figure 3 showed the temperature monitoring and control results. The temperature rise was set to 5ºC in an rabbit thigh. The temporal resolution was 2.27s/frame. To illustrate the function of thermal dose, the base temperature was set to 45ºC. A threshold of 240min was indicated by a dashed line in the thermal dose curve window, while the region where the threshold has been exceed was marked in red.
Discussion and conclusion
MR ARFI and MR thermometry are the two most useful imaging modality for focused ultrasound guidance. In this study, we developed an integrated software based on Gadgetron, which received the k-space data line by line and permit a real-time processing of displacement and temperature mapping. In current implementation, the sequence used for MR-ARFI were spin echo as it suffered less from image distortion. The software is also compatible with EPI sequences when adapting the reconstruction pipeline to EPI procedure. The focus was automatically extracted and labelled in the image. In the future, the registration of the transducer to the image coordinate system would also be integrated into the software using a specifically designed transducer holder. For temperature monitoring, the temperature can be maintained at any targeted value through a temperature MPC algorithm. The temperature curve and corresponding thermal dose would help to evaluate the treatment in real-time and determine the endpoint. In a conclusion, the software is a powerful tool to comprehensively guide and monitor the focused ultrasound therapy.Acknowledgements
This work was supported by the Key Laboratory for Magnetic Resonance and Multimodality Imaging of Guang-dong Province (No. 2014B030301013), the National Natural Science Foundation (Nos. 81327801, 81527901,11504401)References
1. McDannold N, Maier SE, Magnetic resonance acoustic radiation force imaging [J]. Medical physcis, 2008, 35(8): 3748-3758
2. Poorter JD, Wagter CD, Deene YD, Thomsen C, Ståhlberg F, Achten E. Noninvasive MRI thermometry with the proton resonance frequency (PRF) method: in vivo results in human muscle. Magnet Reson Med 1995;33(1):74-81.