3636
Locally and Globally Concatenated Network for MR Image Reconstruction1Philips Research North America, Cambridge, MA, United States, 2Philips Research Hamburg, Hamburg, Germany, 3Department of Radiology, University of Washington, Seattle, WA, United States
Synopsis
Recently, the convolutional neural network (CNN) based reconstruction concept has emerged as a promising implementation of compressed sensing tailored for specific fast imaging applications. The reconstruction performance of such data-driven models may depend on the CNN structure which determines the feature extraction process for sparse representation. In this study, a locally and globally concatenated network is proposed and compared with the residual network as well as the traditional L1-wavelet ESPIRiT. Preliminary experiments on a public knee imaging database showed that the proposed approach provided improved fine structure (e.g. vessel wall) restoration and background noise reduction.
Introduction
Compressed sensing (CS) MRI1 allows highly subsampled acquisition while restoring the diagnostic image quality by properly leveraging the corresponding sparse representation. Data-driven sparse transforms (e.g. dictionary learning, neural network) have shown more powerful CS reconstruction performance in comparison to the universal sparse transforms (e.g. total variation, wavelet)2. In particular, convolutional neural network (CNN) integrated with parallel imaging (PI) or data consistency has recently achieved great success as an end-to-end optimized MR image reconstruction, and offered significantly reduced reconstruction time with more advanced GPU hardware3-6. However, most CNN-based reconstruction models do not take full advantage of the hierarchical features extracted from the subsampled image for sparse representation, resulting in relatively limited performance7,8. In this work, a CNN with local and global concatenations is proposed to enable the full exploitation of hierarchical features across all convolutional layers. Its effectiveness is demonstrated on a public knee dataset.Methods
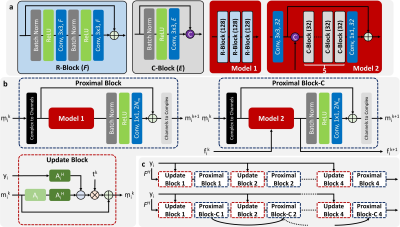
CNN-based Reconstruction Model with Local and Global ConcatenationThe PI-CS reconstruction problem can be solved by interleaved concatenation of two blocks (figure 1c): 1) update block to enforce PI and/or data consistency constraint; 2) proximal block to enforce the sparse representation constraint. Different CNN architectures can be used in the proximal block to create different CNN-based reconstruction models. To adaptively synthesize more effective features from preceding and current features, a stack of local feature concatenation blocks (figure 1a) was exploited to learn a better sparse transform in each proximal block. To further improve the feature connection across different proximal blocks, a global feature concatenation structure was exploited to skip the update block so that the global hierarchical features can be maintained in a holistic way.
MR Experiments
Twenty fully sampled 3D fast spin echo (FSE) knee datasets were downloaded from mridata.org, where 15/2/3 cases were selected respectively for training/validation/testing purpose. A 9.4-fold variable density poisson-disc pattern was used for retrospective random undersampling. L1-norm was used as the loss function during CNN training. CNN training and inference were performed with Tensorflow on an Nvidia Xp GPU. Several different CNN models were trained and compared, including the residual network based model (Proximal Block, Model 1 in figure 1b) and the proposed concatenated network (Proximal Block-C, Model 2 in figure 1b) with/without the global feature concatenation. The total number of convolutional layers and parameters in different CNNs were approximately matched to facilitate a fair comparison. In addition, the L1-wavelet regularized ESPIRiT reconstruction approach9 in the SigPy package (https://github.com/mikgroup/sigpy) was applied as another baseline approach. The peak signal-to-noise ratio (PSNR), normalized root mean square error (nRMSE), and mean structural similarity index (mSSIM) within the tissue region were calculated as quantitative image quality metrics between the fully sampled ground truth and zero-filled/reconstructed images.
Results
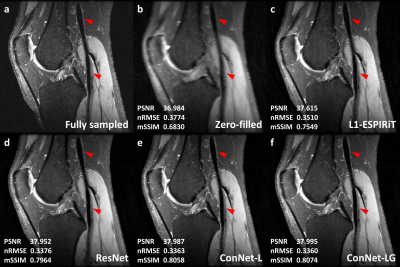
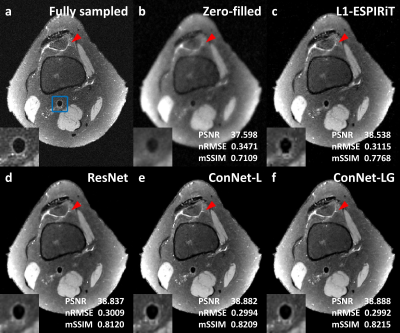
For the three testing cases, the CNN based reconstruction models achieved approximately 0.4dB PSNR increase, 0.01 nRMSE reduction and 0.04 mSSIM improvement compared with the L1-wavelet ESPIRiT (figure 2). Also, an additional 0.01 mSSIM increase was found by using the concatenated network in comparison to the residual network, indicating an improved image structural restoration of the locally and globally concatenated network. Figure 3 showed one example comparison to demonstrate the overall improvement on the vessel wall delineation of popliteal artery with the concatenated network. Figure 4 illustrated a cross-sectional view comparison of the reconstructed vessel wall. The L1-wavelet ESPIRiT might overestimate the wall thickness. The residual network improved the morphological accuracy but with blurring problem. The concatenated network provided the most similar vessel wall morphology and sharpness to the ground truth, while other detailed structures were also better preserved (as shown by red arrows in figure 4). With the global feature concatenation, an improved background noise reduction was observed in comparison to the locally concatenated network (figure 3&4). In addition, the average reconstruction time for each 3D volume was significantly reduced from ~1-hour using L1-wavelet ESPIRiT to ~1-min using CNN based reconstruction with GPU acceleration.Discussion and Conclusion
The locally and globally concatenated network based image reconstruction approach has demonstrated improved detail structural restoration (e.g. vessel wall) and less background noise amplification in highly accelerated 3D FSE knee imaging. The local feature concatenation can better represent the fine structure by actively reusing different levels of features, and the global feature concatenation enables a contiguous mechanism to differentiate the meaningful structure from background noise across all proximal blocks. Therefore, a more powerful sparse representation model can be trained by properly taking advantage of local and global feature fusion. This approach may require further evaluation on its robustness and generalization capabilities in different applications.Acknowledgements
We would like to acknowledge the contributors of mridata.org for sharing the fully sampled knee database, and Dr. Joseph Y. Cheng for sharing his example code on deep learning based reconstruction (https://github.com/MRSRL/dl-cs).References
1. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58(6):1182-95.
2. Yu G, Sapiro G, Mallat S. Solving inverse problems with piecewise linear estimators: From Gaussian mixture models to structured sparsity. IEEE Transactions on Image Processing. 2011;21(5):2481-2499.
3. Sun J, Li H, Xu Z. Deep ADMM-Net for compressive sensing MRI. NIPS 2016, pp. 10-18.
4. Hammernik K, Klatzer T, Kobler E, Recht MP, Sodickson DK, Pock T, Knoll F. Learning a variational network for reconstruction of accelerated MRI data. Magn Reson Med. 2018;79(6):3055-3071.
5. Adler J, Oktem O. Learned primal-dual reconstruction. IEEE Trans Med Imaging. 2018;37(6):1322-1332.
6. Cheng JY, Chen F, Sandino C, Mardani M, Pauly JM, Vasanawala SS. Compressed sensing: from research to clinical practice with data-driven Learning. arXiv:1903.07824v1.
7. Huang G, Liu Z, Van Der Maaten L, Weinberger KQ. Densely connected convolutional networks. IEEE CVPR 2017, pp. 4700-4708.
8. Zhang Y, Tian Y, Kong Y, Zhong B, Fu Y. Residual dense network for image restoration. arXiv preprint arXiv:1812.10477.
9. Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, Vasanawala SS, Lustig M. ESPIRiT--an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magn Reson Med. 2014;71(3):990-1001.
Figures