3596
Deep-learning based motion correction for brain conductivity reconstruction1Philips Research Europe, Hamburg, Germany, 2University of Washington, Seattle, WA, United States
Synopsis
Since tissue conductivity is determined by the numerical second derivative of the phase map, it is particularly susceptible to motion. This abstract investigates the application of deep-learning based methods for retrospective correction of motion artifacts to obtain suitable phase maps as input for conductivity reconstruction. Different types of motion were investigated in the framework of volunteer experiments, revealing that the applied motion correction was indeed capable of improving conductivity reconstruction.
Introduction
Tissue conductivity might be able to serve as important biomarker, particularly for oncologic applications. It is typically reconstructed by numerical calculation of the second derivative of the measured transceive phase map [1], and thus, is very sensitive to any patient motion during acquisition. This study investigated the impact of motion (here: intentional motion in the framework of volunteer brain experiments) on reconstructed conductivity, as well as the possibility to remove motion artefacts by deep-learning techniques prior to conductivity reconstruction. It turned out that this method is able to stabilize conductivity reconstruction for all types of motion investigated.Methods
MR AcquisitionData was acquired on a Philips Ingenia 3T scanner (Best, The Netherlands) using a head-neck receive coil. A T2-weighted multi-slice turbo spin-echo sequence was used with TR = 3 s, TSE factor = 16, TE = 80 ms, echo spacing = 9.4 ms. Geometric parameter were chosen as follows: FOV 230 x 182 mm2, 0.55 x 0.65 mm2 voxel size in 28 slices with 4 mm slice thickness. Following written informed consent obtained according to local Institutional Review Board guidelines, a healthy volunteer performed deliberate, specified motion during data acquisition to induce varying severity of motion artifacts within the resulting images. These included remaining as still as possible (as a baseline experiment), performing repeated coughing, continuous leg shaking, continuous head movement, performing a single shift in head position, and performing continuous eye movement.
Motion Correction
Motion artifacts in the input data were corrected separately for magnitude and phase. To this end, two tailored fully convolutional networks were trained on a dataset of MR image pairs with and without artificially generated motion artifacts. The training dataset was based on 16 T2-weighted (multi-2D spin echo, magnitude data only) whole-brain scans obtained in 16 patients. Following informed patient consent, all data was anonymized, and all images were deemed to be artifact-free by an experienced, blinded neuroradiologist. Synthetic phase images were created using a 3rd-order 2D polynomial with randomly selected coefficients and added to the magnitude data. For both networks, artifact-corrupted magnitude and phase was provided as input, but only artifact-free magnitude (network 1) or phase (network 2) data was used as target. Artifact simulation was realized using a previously described pipeline [2]. A U-Net with 19 convolutional layers and 5 down-/up-sampling operations in the contracting/expanding path was implemented using the PyTorch framework. After training, both networks were applied to magnitude and transceive phase images to correct for motion artifacts. The transceive image was used as input to the EPT reconstruction as outlined below, while the magnitude images are only required here to illustrate anatomical structures and artefacts.
EPT Reconstruction
Conductivity $$$\sigma$$$ was reconstructed by numerical differentiation from the uncorrected and corrected transceive phase $$$\phi$$$, respectively, according to $$$\sigma = \nabla^2 \phi / (2\mu\omega)$$$ (with vacuum permeability $$$\mu$$$ and Larmor frequency $$$\omega$$$). The kernel of the numerical differentiation as well as of the subsequent denoising filter was locally shaped to exclude tissue boundaries (as identified from the magnitude image) to avoid the otherwise occurring boundary artefacts [1].
Results
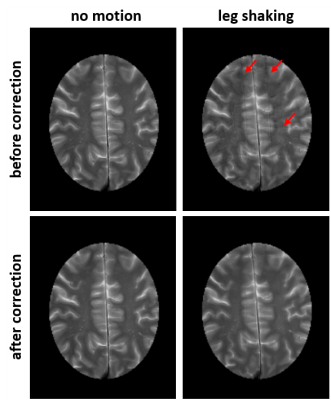
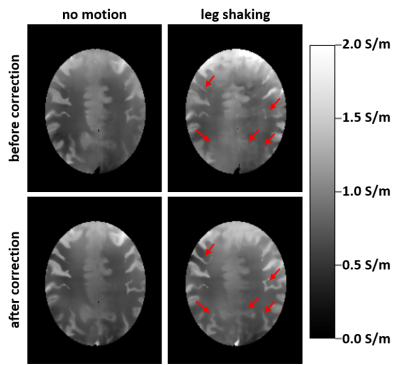
Anatomical images before and after U-net correction for the baseline experiment (no motion) and continuous leg shaking are presented in Fig. 1. The characteristic ghosting artifacts due to subject motion are well visible before the correction and significantly reduced after correction.Figure 2 presents the derived conductivity maps for the same cases as in Fig. 1. Structures of the cortex are blurred or obstructed in the motion case without correction. After correction, much more detail is recovered in the conductivity map. As expected, no significant difference is visible before and after correction for the case without motion.
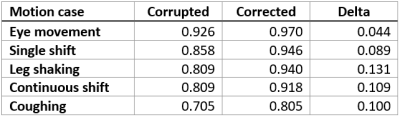
Correlation coefficients of the conductivity maps of the corrupted and corrected motion cases calculated with respect to the corrected no motion case (applying corresponding elastic image registration [3] to correct for inter-scan motion and accompanying shifting of structures) are presented in Tab. 1. After network processing, the correlation to the baseline experiment was improved for all motion cases.
Discussion
In our experiments, the resulting conductivity maps showed an improved correlation to the baseline map independent of the motion case, demonstrating the versatility of the approach. Moreover, the correction network did not decrease the quality of the conductivity map in an acquisition without motion of the subject.Conclusion
Retrospective motion artifact removal in MR phase maps with deep learning methods substantially improve the quality of EPT reconstructed brain conductivity maps. This is noteworthy, as the conductivity is derived from the second order derivative, making this type of reconstruction particularly sensitive to motion artifacts. The proposed method hence appears to facilitate enhanced motion robust conductivity mapping.Acknowledgements
No acknowledgement found.References
[1] U. Katscher et al., Electric properties tomography: Biochemical, physical and technical background, evaluation and clinical applications. NMR Biomed. 2017; 30:3729
[2] K. Sommer, et al., Correction of motion artifacts using a multi-resolution fully convolutional neural network, Proc. Intl. Soc. Mag. Reson. Med. 26, #1175, 2018.
[3] S. Kabus and C. Lorenz, “Fast elastic image registration,” in Medical Image Analysis For The Clinic - A Grand Challenge, 2010, pp. 81–89
Figures