3576
Deep Learning Global Schedule Optimization for Chemical Exchange Saturation Transfer MR Fingerprinting (CEST-MRF)1Radiology, Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 2Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Synopsis
Chemical exchange saturation transfer MR fingerprinting (CEST-MRF) enables quantification of multiple tissue parameters. Optimization of the acquisition schedule can improve tissue discrimination and reduce scan times but is highly challenging because of the large number of acquisition and tissue parameters. The goal of this work is to demonstrate a scalable deep learning based global optimization method that provides schedules with improved discrimination. The benefits of our approach are demonstrated in an in vivo mouse tumor model.
Introduction
Chemical exchange saturation transfer (CEST) MRI is a powerful molecular imaging technique1–3 but its clinical translation is hindered by the qualitative nature of the image contrast. Recently, we’ve introduced a CEST MR Fingerprinting (MRF) sequence4 that simultaneously yields quantitative relaxation (water T1/T2) and CEST (exchange rates, volume fractions) parameters. To improve tissue discrimination and shorten acquisition times, the acquisition schedule should be optimized5,6 but optimization of a large number of parameters is a challenging problem. The majority of alternative approaches to date optimized only a small set of acquisition parameters and tissue parameter values7,8 representative of healthy tissues. As such, the schedules obtained are unlikely to be optimal for pathological cases where the parameter values can deviate significantly from those of healthy tissue.To overcome these issues, in this work we build on our Schedule Optimization Network (SCONE)9 and describe an algorithm for global optimization of high dimensional MRF schedules and tissue parameters. The utility of our method is demonstrated in a mouse glioblastoma tumor model and enables improved quantification of 6 simultaneous tissue parameters.
Methods
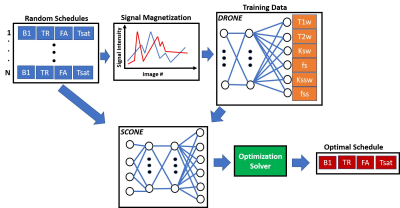
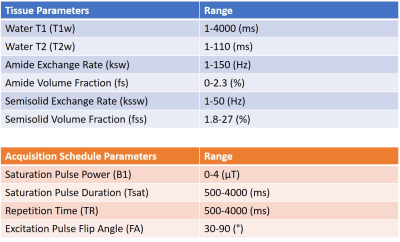
Tissue and Schedule SamplingAn outline of the method is shown in Figure 1. Instead of solely optimizing over three tissue types (typically white matter, grey matter and cerebrospinal fluid), a set of 4000 distinct tissue parameter combinations was drawn from the 6-dimensional tissue parameter space using latin hypercube sampling10. The six parameters used were: water T1 (T1w), water T2 (T2w), amide exchange rate (ksw) and volume fraction (fs) as well semisolid exchange rate (kssw) and volume fraction (fss). A set of 1000 schedules were similarly drawn from the acquisition parameter space by sampling the saturation pulse power (B1), saturation pulse length (Tsat), repetition time (TR) and the flip angle of the excitation pulse (FA). The ranges for the tissue and acquisition parameters are shown in Figure 2.
Network Training
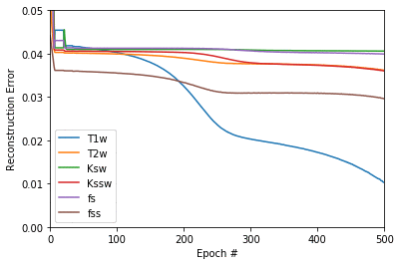
Each of the acquisition schedules drawn was used to simulate a CEST-MRF acquisition for the 4000 tissues parameter values by numerically solving the Bloch-McConnell equations. The resulting signal magnetizations were used as training data for the DRONE11 neural network which was trained for 500 epochs with a batch size of 1000, learning rate of 0.0001 and 20% validation set . The training cost of each tissue (T1w, T2w etc.) represented the reconstruction error for a given acquisition schedule (Figure 3). Since the initialization and training parameters were kept constant between schedules, the acquisition schedule was the only contributor to the resulting error which enables comparison between different schedules.
The acquisition schedules and their calculated training costs were used to train a second neural network to determine a functional mapping between the acquisition schedule space and the reconstruction error. As we’ve previously demonstrated11, an accurate functional mapping can be obtained despite the use of a sparse training dataset.
Schedule Optimization
Since evaluation of the reconstruction error of a schedule with the network is ~2000 times faster than conventional MRF-CEST simulation, a larger fraction of the search-space can be explored in a given compute time yielding improved optima. The network was used in combination with the patternsearch solver in MATLAB to find the schedule with the minimum reconstruction error. The optimization was repeated 50 times with randomly selected schedules as well as the schedule used in the original MRF-CEST paper4 and run to convergence. Among the resulting 50 optimized schedules, the one with the lowest cost was used for the in vivo acquisition.
In Vivo Imaging
All animal procedures were approved by the institutional committee. U87 tumors were implanted in the brain of the mouse which was imaged at 11 days after implantation, using a 7T preclinical MRI (Bruker, Germany). The CEST-MRF data was reconstructed by a DRONE network trained on a 400,000 entries training dictionary selected from the same ranges shown in Figure 2.
Results
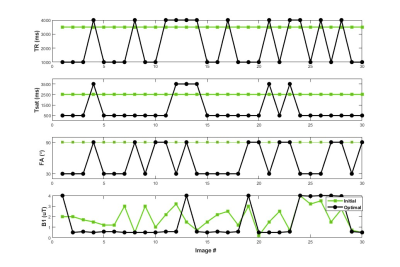
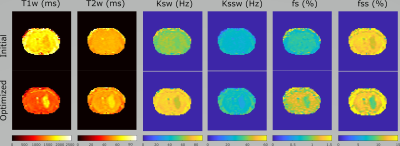
The initial and optimized schedules are shown in Figure 4 and the resulting tissue maps are shown in Figure 5. The original schedule required a scan time of 105 seconds and showed poor discrimination between the tumor and the contra-lateral tissues. The optimized schedule was 37% shorter (66 s) and provided improved tissue discrimination between the different the tissue types. The increased T1 and T2 and reduced volume fractions shown for the optimized schedule are consistent with the presence of edema in the tumor as shown by other groups12,13.Discussion/Conclusion
Combined with multiple initializations, SCONE enables global optimization of high dimensional acquisition schedules. Once trained, the acquisition constraints can be changed to suit different pulse sequences without the need to retrain the network. Our method is highly parallelizable and can benefit from efficient implementations6 of the CEST-MRF simulation code. Efficiently sampling a larger set of tissue and acquisition parameters is expected to significantly improve the optimization and is the focus of ongoing research. Because it uses a range of tissues, the schedules optimized by SCONE-CEST can better discriminate pathologies than those optimized for a small range of healthy tissue values and are thus better suited for clinical adoption.Acknowledgements
O.P. acknowledges funding from the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement No. 836752 (OncoViroMRI).References
1. Ward, K., Aletras, A. & Balaban, R. S. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J. Magn. Reson. 143, 79–87 (2000).
2. Ma, B. et al. Applying amide proton transfer-weighted MRI to distinguish pseudoprogression from true progression in malignant gliomas. J. Magn. Reson. Imaging 44, 456–462 (2016).
3. Zhou, J., Payen, J.-F., Wilson, D. A., Traystman, R. J. & van Zijl, P. C. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat. Med. 9, 1085 (2003).
4. Cohen, O., Huang, S., McMahon, M. T., Rosen, M. S. & Farrar, C. T. Rapid and quantitative chemical exchange saturation transfer (CEST) imaging with magnetic resonance fingerprinting (MRF). Magn. Reson. Med. 80, 2449–2463 (2018).
5. Cohen, O. & Rosen, M. S. Algorithm comparison for schedule optimization in MR fingerprinting. Magn. Reson. Imaging 41, 15–21 (2017).
6. Perlman, O. et al. CEST MR-Fingerprinting: practical considerations and insights for acquisition schedule design and improved reconstruction. Magn. Reson. Med. 83, 462–478 (2020).
7. Zhao, B. et al. Optimal experiment design for magnetic resonance fingerprinting: Cramer-Rao bound meets spin dynamics. IEEE Trans. Med. Imaging 38, 844–861 (2018).
8. Lee, P. K., Watkins, L. E., Anderson, T. I., Buonincontri, G. & Hargreaves, B. A. Flexible and efficient optimization of quantitative sequences using automatic differentiation of Bloch simulations. Magn. Reson. Med. (2019).
9. Cohen, O. MR Fingerprinting Schedule Optimization Network (MRF-SCONE). in Proceedings of the International Society of Magnetic Resonance in Medicine 4531 (May 11-May 16).
10. Park, J.-S. Optimal Latin-hypercube designs for computer experiments. J. Stat. Plan. Inference 39, 95–111 (1994).
11. Cohen, O., Zhu, B. & Rosen, M. S. MR fingerprinting deep reconstruction network (DRONE). Magn. Reson. Med. 80, 885–894 (2018).
12. Lescher, S. et al. Quantitative T1 and T2 mapping in recurrent glioblastomas under bevacizumab: earlier detection of tumor progression compared to conventional MRI. Neuroradiology 57, 11–20 (2015). 13. Hammoud, M. A., Sawaya, R., Shi, W., Thall, P. F. & Leeds, N. E. Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J. Neurooncol. 27, 65–73 (1996).
Figures