3558
MRI raw k-space mapped directly to outcomes: A study of deep-learning based segmentation and classification tasks1GE Healthcare, Bangalore, India, 2GE Global Research, Niskayuna, NY, United States
Synopsis
Most of the advancements with deep learning have come from mapping the reconstructed MRl images to outcomes (e.g. tumor segmentation, survival rate, pathology risk map). In this work, we present methods to arrive at critical medical imaging tasks like segmentation, classification directly from raw k-space data without image reconstruction. We specifically demonstrate that from k-space MRI data, we can perform hippocampus segmentation as well as detection of motion affected scans with similar performance to that obtained from imaging data. We also demonstrate that such an approach is more resilient to localized artifacts (e.g signal loss in hippocampus due to metal).
Introduction
Deep learning has improved reconstruction of MR images from raw-data and become indispensable part of workflow chain [1-4] . However, one of the questions that is still unanswered is the “information loss” due to the reconstruction process and its effect on outcomes of interest. Previous efforts have seen improvements when raw-data is translated to “raw image” and convolutional neural networks (CNN) used to accomplish segmentation tasks5,6. In this work, we directly map raw MRI k-space data to clinical outcomes using AUTOMAP1 construct; thereby bypassing all the reconstruction steps altogether (Fig.1). We demonstrate efficacy of the method in two common scenarios: Segmentation (using hippocampus segmentation in brain) and Classification (using head motion detection). We also ascertain the advantages of raw data-based outcomes by simulating artifacts in the image and ascertain its performance in hippocampus segmentation.Methods
Subjects: MRI data from three different clinical sites.Cohort A.: Used for Hippocampus Segmentation, 855 clinically negative subjects ;
Cohort B: Used for motion detection, 441 patients (stroke, tumors, tremors) , two sites.
All studies approved by appropriate IRB.
MRI Scanner and Acquisition:
Cohort A: GE 3T Discovery MR750 scanner, dedicated head coil, 2D SSFSE data.
Cohort B: 1.5T GE SignaHDxt MRI scanner, 8-channel brain and head-neck-spine coils, Multiple MRI protocols (T1W, T1W+CE, T2W, FLAIR) and orientations.
Data for both Cohort A and Cohort B were acquired with Cartesian k-space trajectory.
Ground-truth (GT):
A. For Hippocampus Segmentation: Ground-truth data was obtained by registering MNI brain atlas to patient data using non-rigid registration.
B. Motion Detection (See Figure 2): Trained radiologist labelled each MRI volume as belonging to:
a. Class-1 images with no motion artifacts present or images with motion present but acceptable for diagnostic purpose and
b. Class-2 images with motion artifacts present & are not acceptable for diagnostic purposes. (See Fig.2).
Raw K-space Data: For all the experiments described below, we re-sampled the data to 128x128 matrix in-plane. Next raw data was simulated by taking the Fourier transform of the magnitude data and obtaining the complex k-space data.
Deep Learning (DL) Experiments
For all the experiments, we used AUTOMAP / UNet architecture /classification network as described in literature [1, 7,8]. Typical parameters: Epochs = 100, optimizer = rmsprop, batch_size = 32. See fig. 2 and 3.Train and test split:
A. Hippocampus Segmentation: 30000 train images and 1000 test images.
B. Motion Detection: 15000 train images, 1500 test images
DL Experiments:
Experiment #1: AUTOMAP based image reconstruction. Input: Raw k-space, output: MR image, mean-squared error (MSE) loss.
Experiment #2: 2D UNet segmentation, dice loss. Input: MR image, output: hippocampus segmentation mask. Evaluated on test data reconstructed with Experiment #1.
Experiment #3 (raw data to segmentation mask): AutoMap + UNet based image segmentation: Input: raw data, output: hippocampus segmentation mask (See Figure 3a).
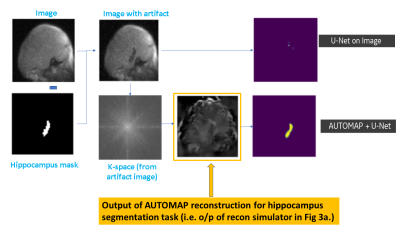
Experiment #4 (Impact of artifact on hippocampus segmentation, See Figure 3b.): Simulate metal artifact by randomly modifying the signal intensity between 5% to 50% within hippocampus mask and add/subtract it from image data. Obtain raw k-space data from artifact images. Perform segmentation task as described in Experiment #2 and #3.
Experiment #5 (image data to classification task): CNN based classification network8: Input: image data, output: motion labels (Class-1, class-2).
Experiment #6 (raw data to classification task): AutoMap+ UNet based image classification: Input: raw data, output: motion labels (Class-1, class-2)
Evaluation Criteria:All evaluation done only for test cases.
Experiment #1: mean absolute intensity error (mae),
Experiment #2 , 3 and 4: Dice overlap,
Experiment #5 and 6: Area under ROC curve (AUC).
Results and Discussion
Overall, for experiment #1, mae is ~2% (Figure 4A). Dice overlap score for hippocampus segmentation is similar (~83%) for both the image based and raw data-based methods even on AUTOMAP reconstructed data (figure 4B and 4C). Since metal artifact is localized in hippocampus region, CNN -based UNet segmentation is affected by artifact signal intensity drop (10%) and unable to predict hippocampus (Figure 5). On the contrary, raw data-based approach is still able to segment the hippocampus, since artifact is not localized in k-space and spread out. The intermittent representation of hippocampus segmentation manifold learnt by the raw data approach (figure 5) does not exactly represent brain anatomy but is more abstract. This abstract feature space allows raw data-based analytics to provide more resilience to imaging artifacts when performing certain medical imaging tasks. Overall, we noticed that image-based segmentation worked in only 13% of cases with induced metal artifacts in test cases, while in case of raw data-based methods, the success rate was ~ 55%. Signal intensity drop > 30% caused failure even with raw data mapping. For head motion detection classification task, the AUC was 98% for experiment #5 while for raw data-based classification (for experiment #6), accuracy dropped marginally to ~ 96%.Conclusion
In this work, we demonstrated that direct raw-data to outcome-based measures are possible with AUTOMAP based network construct. We notice almost similar performance between image based and raw data-based imaging tasks such as segmentation and classification. However, when there are localized imaging artifacts, we observed that raw-data based methods are much better adept at handling these artifacts; thereby providing a more robust outcome measure compared to image-based methods.Acknowledgements
No acknowledgement found.References
1) Zhu, Bo, et al. "Image reconstruction by domain-transform manifold learning." Nature 555.7697 (2018): 487
2) Hyun, Chang Min, et al. "Deep learning for undersampled MRI reconstruction." Physics in medicine and biology (2018).
3) Deep Learning For Sparse MR Reconstruction in Glioma Patients,Peter D Chang, Michael Z Liu, Daniel S Chow, Melissa Khy, Christopher G Filippi, Janine Lupo, and Christopher Hess
4) Schlemper, Jo, et al. "A deep cascade of convolutional neural networks for dynamic MR image reconstruction." IEEE transactions on Medical Imaging 37.2 (2018): 491-503.
5) Schlemper J. et al. (2018) Cardiac MR Segmentation from Undersampled k-space Using Deep Latent Representation Learning. In: Frangi A., Schnabel J., Davatzikos C., Alberola-López C., Fichtinger G. (eds) Medical Image Computing and Computer Assisted Intervention – MICCAI 2018. MICCAI 2018. Lecture Notes in Computer Science, vol 11070. Springer, Cham
6) Huang Q., Chen X., Metaxas D., Nadar M.S. (2019) Brain Segmentation from k-Space with End-to-End Recurrent Attention Network. In: Shen D. et al. (eds) Medical Image Computing and Computer Assisted Intervention – MICCAI 2019. MICCAI 2019. Lecture Notes in Computer Science, vol 11766. Springer, Cham
7) U-Net: Convolutional Networks for Biomedical Image Segmentation, Olaf Ronneberger, Philipp Fischer, Thomas Brox, Medical Image Computing and Computer-Assisted Intervention (MICCAI), Springer, LNCS, Vol.9351: 234--241, 2015
8) A. Sreekumari, D. Shanbhag, D. Yeo, T. Foo, J. Pilitsis, J. Polzin, U. Patil, A. Coblentz, A. Kapadia, J. Khinda, A. Boutet, J. Port, I. Hancu, American Journal of Neuroradiology Feb 2019, 40 (2) 217-223;
Figures
Figure 3.
a. Raw data to outcome tasks setup. Input to architecture is concatenated vector of real and imaginary parts of k-space (image size = NxN). The dense layers act as recon. function approximators, followed by convolutional layers that map the intermediate output to abstract space. This is followed by a U-NET (i.e. a FCN architecture) which produces the task output (hippocampus seg.). The UNet can be replaced with classification network based on task.
b. Simulate imaging artifacts : Blooming / susceptibility signal loss is simulated using signal changes within hippocampus mask
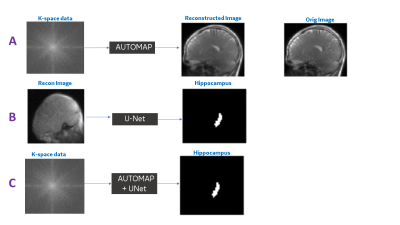
Figure 4. Example output from Experiment #1 (A), Experiment #2 (B) and Experiment #3 (C).
A: As expected, the AUTOMAP trained with MSE loss has the smoothing characteristics in the output image data (reconstruction error = 2%).
B and C: Overall, we notice that hippocampus segmentation quality is similar for both raw data and image data based approaches