3533
Association of MRI-derived radiomic biomarker with disease-free survival in patients with early-stage cervical cancer
Jin Fang1, Bin Zhang1, Shuo Wang2, and Shuixing Zhang1
1The First Affiliated Hospital of Jinan University, Guangzhou, China, 2Key Laboratory of Molecular Imaging, Institute of Automation, Chinese Academy of Sciences, Beijing, China
1The First Affiliated Hospital of Jinan University, Guangzhou, China, 2Key Laboratory of Molecular Imaging, Institute of Automation, Chinese Academy of Sciences, Beijing, China
Synopsis
Radiomics is a promising methodology that automatically extracts high-dimensional features from imaging data for supplementary evaluation of prognosis. Herein, we developed radiomic signature based on pretreatment MRI, which can be used as a biomarker for risk stratification for disease-free survival (DFS) in patients with early-stage cervical cancer. This study provides a non-invasive and cost-effective preoperative predictive tool to identify the early stage cervical cancer patients with high possibility of recurrence or metastasis; and they may help to determine whether more intensive observation and aggressive treatment regimens should be administered, aim at assisting clinical treatment and healthcare decisions.
Introduction
For early-stage cervical cancer, only about 70% patients have a 5-year disease-free survival (DFS) [1]. Previous studies have identified the depth of invasion, lymph node metastasis (LNM) and lymphovascular space invasion (LVI) as risk factors for recurrence and metastasis in cervical cancer patients [2,3]. These risk factors are determined through random sampling biopsy or surgery, which have limitations, including procedure-related complications, sampling error, and interobserver variability [4]. In this scenario, non-invasively prognostic biomarkers that allow assessment of tumor heterogeneity are warranted. It has been conclusively shown that radiomic features could be used to diagnose precisely, evaluate treatment response, and predict survival in various types of cancers [5-9]. Therefore, this study aims to develop a radiomic score using pre-treatment MRI to estimate 3-year DFS in patients with early-stage cervical cancer, and to further construct a combined model combing the radiomic score and the clinicopathological features for the individual prediction of DFS.Methods
A total of 248 patients with early-stage cervical cancer underwent radical hysterectomy were included from two institutions between January 1, 2011 and December 31, 2017, their MR imaging data, clinicopathological data and DFS data were collected. Patients data were randomly divided into the training cohort (n = 166) and the validation cohort (n=82). Radiomic features were extracted from the pre-treatment T2-weighted (T2w) and contrast-enhanced T1-weighted (CET1w) MR imaging for each patient. Least absolute shrinkage and selection operator (LASSO) regression and Cox proportional hazard model were applied to construct radiomic score (Rad-score). According to the cutoff of Rad-score, patients were divided into low- and high- risk groups. Pearson’s correlation and Kaplan-Meier analysis were used to evaluate the association of Rad-score with DFS. A combined model incorporating Rad-score, lymph node metastasis (LNM) and lymphovascular space invasion (LVI) by multivariate Cox proportional hazard model was constructed to estimate DFS individually.Results
Higher Rad-scores were significantly associated with worse DFS in the training and validation cohorts (P<0.001both)(Figure 1). The radiomic score yielded an AUC of 0.822 (95%CI: 0.765-0.882), the clinicopathological model achieved an area under the receiver operating characteristic curve(AUC)of 0.666 (95%CI: 0.595-0.742). However, the combined model shows no significant improvement. (Figure 2). Figure 3 shows two representative patients with distinctly different DFS time (14 months vs 64 months), although they had almost the same clinicopathological features, their Rad-scores (2.046 vs 0.237) were significantly different.Conclusions
This study provides a noninvasive and pretreatment prognostic biomarker for the DFS of cervical cancer based on MRI. Moreover, for each cervical cancer patient, the radiomic score can predict the hazard risk of the patient being disease-free and stratify the patient into high-risk and low-risk groups. The study may offer some important insights into precise treatment, providing valuable guidance for clinical physicians regarding the treatment strategies including radical hysterectomy or chemoradiation in early-stage cervical cancer patients.Acknowledgements
NoneReferences
- Moore KN, Java JJ, Slaughter KN, Rose PG, Lanciano R, DiSilvestro PA, et al. Is age a prognostic biomarker for survival among women with locally advanced cervical cancer treated with chemoradiation? An NRG Oncology/Gynecologic Oncology Group ancillary data analysis. Gynecol Oncol. 2016; 143: 294-301.
- Creasman WT, Kohler MF. Is lymph vascular space involvement an independent prognostic factor in early cervical cancer? Gynecol Oncol. 2004; 92: 525-9.
- Skret-Magierlo JE, Soja PJ, Skret A, Kruczek A, Kaznowska E, Wicherek L. Perineural space invasion in cervical cancer (FIGO IB1-IIB) accompanied by high-risk factors for recurrence. J Cancer Res Ther. 2014; 10: 957-61.
- Choe J, Lee SM, Do KH, Lee JB, Lee SM, Lee JG, et al. Prognostic value of radiomic analysis of iodine overlay maps from dual-energy computed tomography in patients with resectable lung cancer. Eur Radiol. 2019; 29: 915-23.
- Zhang B, Tian J, Dong D, Gu D, Dong Y, Zhang L, et al. Radiomics Features of Multiparametric MRI as Novel Prognostic Factors in Advanced Nasopharyngeal Carcinoma. Clin Cancer Res. 2017; 23: 4259-69.
- Park H, Lim Y, Ko ES, Cho HH, Lee JE, Han BK, et al. Radiomics Signature on Magnetic Resonance Imaging: Association with Disease-Free Survival in Patients with Invasive Breast Cancer. Clin Cancer Res. 2018; 24: 4705-14.
- Huang Y, Liu Z, He L, Chen X, Pan D, Ma Z, et al. Radiomics Signature: A Potential Biomarker for the Prediction of Disease-Free Survival in Early-Stage (I or II) Non-Small Cell Lung Cancer. Radiology. 2016; 281: 947-57.
- Liu Z, Wang S, Dong D, Wei J, Fang C, Zhou X, et al. The Applications of Radiomics in Precision Diagnosis and Treatment of Oncology: Opportunities and Challenges. Theranostics. 2019; 9: 1303-22.
- Liu Z, Li Z, Qu J, et al. Radiomics of multi-parametric MRI for pretreatment prediction of pathological complete response to neoadjuvant chemotherapy in breast cancer: a multicenter study. Clin Cancer Res. 2019; [Epub ahead of print].
Figures
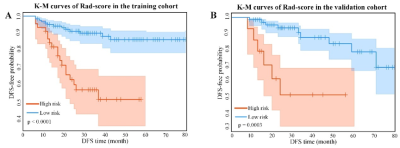
Figure 1. Kaplan-Meier analysis of the Rad-score. A)
Kaplan-Meier curves of the Rad-score in the training cohort. Vertical lines
indicate censored data. Shadows represent 95% CI. B) Kaplan-Meier curves of the
Rad-score in the validation cohort. DFS: disease-free
survival. CI: confidence interval.
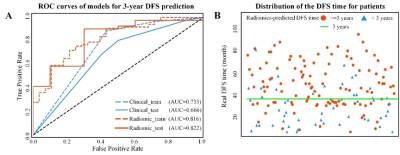
Figure 2. A) ROC curves of the two models for 3-year
DFS probability prediction. B) Distribution of the DFS time for patients. The
red circle represents patients who are predicted to have DFS time larger than 3
years by the Rad-score, and the blue triangle represents patients who are
predicted to have DFS time less than 3 years by the Rad-score. Most patients
who are predicted to have DFS time larger 3 years distribute above the patients
who are predicted to have DFS time less than 3 years. ROC: receiver-operating characteristic; DFS: disease-free
survival.
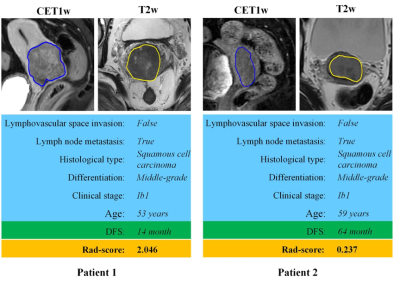
Figure 3. MR images of two patients with significantly
different DFS time. Although patient 1 and patient 2 have similar clinicopathological
characteristics, their Rad-score are different and discriminative. DFS: disease-free
survival.