3529
Combined clinical parameters and Magnetic Resonance Images for prostate cancer detection1Peking University, Beijing, China, 2Peking University First Hospital, Beijing, China
Synopsis
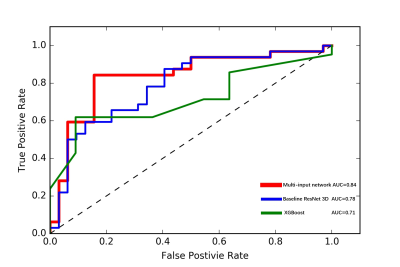
In the wake of population aging, prostate cancer has become one of the most important diseases in elderly men. The low specificity in only image-based diagnosis may lead to unnecessary biopsies. Therefore, clinicians need to consider other variables to make diagnosis, such as age, PSA, and prostate volume. In this study we developed a novel 3D CNN model which combined clinical parameters and MR images for differentiating benign and malignant prostate lesions. The area under the receiver operating characteristics (ROC) of our proposed model (0.84) is significantly higher than that of traditional prediction model (0.71, P < 0.001).
Introduction
In the wake of population aging, prostate cancer has become one of the most important diseases in western elderly men, especially in United State.[1] Multi-parametric (mp-MRI) is the most common used non-intrusive technique to diagnosis prostate cancer [2]. The Prostate Imaging Reporting and Data System (PI‐RADS) was designed to standardize mp‐MRI sequence acquisition and reporting and increase the sensitivity of PCa identification by introducing a 5‐score strategy to reveal the likelihood of clinically significant disease [3]. But the specificity is still poor which may lead to unnecessary biopsies. Therefore, clinicians usually need to consider other indicators to determine the cancer probability of patients, such as age, PSA, and prostate volume. However, the accuracy of the decision largely depends on the experience of the radiologist, and interobserver variation also exists. Therefore, an advanced method incorporating mp‐MRI and clinical indicators for discrimination of benign from malignant lesions in the prostate is necessary.Method
Image Acquisitions:Clinical routine MRI was conducted on a cohort of 150 subjects, including healthy subjects, and prostate cancer patients. The imaging was performed on a 3.0 T Ingenia system (Philips Healthcare, the Netherlands), with acquisition of T2-weighted, diffusion-weighted and dynamic contrast-enhanced images. DWIs were obtained in the axial, sagittal, and coronal planes with TR: 3970 ms; TE: 68 ms; FOV: 240 mm×240 mm; matrix: 184×184; slice thickness: 4 mm with no gap; two b values (b0, b1000).
Study Population:
We performed this retrospective study with permission from the local Institutional Ethical Committee. The need for informed consent was waived. Between 2013 and 2016, 150 consecutive patients were selected. Among them, 87 patients were confirmed to have PCa by biopsy, and the other 63 patients had not had cancer detected by serial biopsy or during long-term follow-up. For each subject, 10 typical slices which contained the whole prostate in high b value DWIs were selected in the image datasets. And full data on PI-RADS score, PSA, age, digital-rectal examination (DRE), prostate volume (PV) were selected in the Clinical Parameters datasets. (70 subjects as the training set, 40 subjects as the Verification set and 40 subjects as the testing set).
Algorithm description:
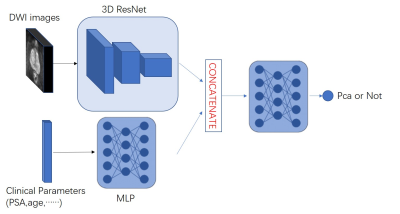
In the paper, we proposed a new deep network framework. As showed in Fig. 1, the framework accept both the numerical data along with image data. In order to build our multi-input network, we will need two branches: The first branch will be a simple Multi-layer Perceptron (MLP) designed to handle the Clinical Parameters inputs. The second branch will be a 3D Convolutional Neural Network to extract the image feature. Then, we concatenated the final dense layer of the 3D CNN and the final layer of MLP together, and integrated the image features and Clinical Parameters by three fully connected layers. The loss function of the Network we proposed in this paper is binary cross entropy function.The network was implemented using Keras with the Adam optimizer using a learning rate of 1e−5. And the network was trained and tested on an NVIDIA GTX1080 GPU with 8 GB of memory.
Result
We evaluated the performance of the 3D-ResNet in the binary classification of the Pca by use of receiver operating characteristics (ROC) analysis. We used the area under the ROC curve (AUC) as the figure of merit of the classification performance.Figure 2 shows the AUC values and ROC curves that indicate the performance of the deep-learning models in the classification of Pca. The ROC analysis showed that the AUC of the multi-input model (0.84) was significantly higher than the traditional XGBoost prediction model(0.71) and baseline ResNet3D model(0.78).(p<0.01)
Discussion and Conclusion
In this study we developed a novel 3D CNN model which combined clinical parameters and MR images for detecte prostate cancer. Our key contribution is followed: (1) in order to prevent the loss of important information, we developed a 3D ResNet by replacing the originally proposed ResNet, which can capture the spatial features of 3D prostate structure more comprehensively and accurately. (2) Inspired by the clinical workflow for prostate cancer detection, the structure of 3D ResNet was modified to deal with multiple inputs and single outputs, which can fuse depth image features with traditional clinical features. The result shows that our model is reliable for the diagnosis of prostate cancer, and the predicted results can be used as the “third eye” for clinical diagnosis In our future work, we hope to get better classification results by adding more clinical parameters such as PSA velocity and adding more imaging sequences such as T2WI and DCE.Acknowledgements
No acknowledgement found.References
[1]Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014[J]. CA: a cancer journal for clinicians, 2014, 64(1): 9-29.
[2] Villers A, Lemaitre L, Haffner J, et al. Current status of MRI for the diagnosis, staging and prognosis of prostate cancer: implications for focal therapy and active surveillance[J]. Current opinion in urology, 2009, 19(3): 274-282.
[3]Radtke J P , Wiesenfarth M , Kesch C , et al. Combined Clinical Parameters and Multiparametric Magnetic Resonance Imaging for Advanced Risk Modeling of Prostate Cancer—Patient-tailored Risk Stratification Can Reduce Unnecessary Biopsies[J]. European Urology, 2017:S0302283817302671.