3521
Multi-Contrast Hippocampal Subfield Segmentation for Ultra-High Field 7T MRI Data using Deep Learning1Department of Health Science and Technology, Aalborg University, Aalborg, Denmark, 2Centre for Advanced Imaging, University of Queensland, Brisbane, Australia, 3School of Information Technology and Electrical Engineering, University of Queensland, Brisbane, Australia
Synopsis
Ultra-high field 7T MRI and the utilization of multiple MRI contrasts potentially enable a superior hippocampal subfield segmentation. A residual-dense fully convolutional neural network based on U-net, including a dilated-convolutional-block was implemented for hippocampal subfield segmentation. Two data sets were combined for training and mean DSC of 0.7723 was obtained. DSC was higher for larger subfields, which were undersegmented, while smaller subfields were oversegmented. Results were comparable to the atlas-based method ASHS, while providing a substantially faster processing time.
Introduction
The volume of hippocampal subfields are differentially affected in Alzheimer's Disease (AD) and are effective biomarkers.1,2 Analysis of neurodegeneration in hippocampal subfields in Magnetic Resonance (MR) images can potentially allow for an AD diagnosis in its presymptomatic stage.3 Automated methods can achieve consistent segmentations that are less affected by inter- and intra-rater reliability and are substantially more time-efficient than manual segmentation4-6, but need to provide comparable or superior performance to be clinically applicable. Ultra-high field 7 Tesla [T] images result in high resolution and signal-to-noise-ratio, and when combined with multiple MR contrasts, it enables potentially superior hippocampal subfield segmentation.1,4,6,7 The aim of this study was to develop a deep neural network for segmentation of hippocampal subfields using multi-contrast 7T MRI.Methods
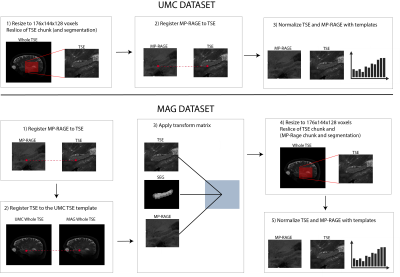
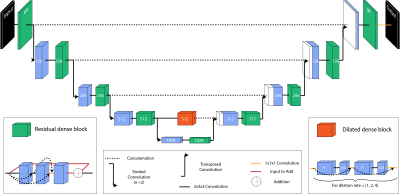
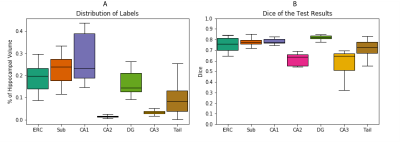
Two publicly available data sets8, Universitair Medisch Centrum (UMC) Utrecht9 and Magdeburg10, each including T1w (MP-RAGE) and T2w (TSE) scans of 7T MR brain images, were combined through preprocessing and resulted in a data set of 111 hippocampi. A fully reproducible preprocessing pipeline, illustrated in Figure 1, was implemented using Nipype11. This ensured identical resolution and size of volumes across the data sets, and that both MR contrasts and the segmentation for each hippocampi were located in the same space. Both data sets contained a manual segmentation and labelling of the same subfields including Entorhinal Cortex (ERC), Subiculum (Sub), Cornu Ammonis (CA)1, CA2, CA3, Tail, and Dentate Gyrus (DG), which contained CA4.A test set of 10 % (n = 11) was randomly selected from the data set. The remaining data were left-right flipped (n = 200) and randomly split into a training set of 90 % (n = 180) and a validation set of 10 % (n = 20).The proposed method is a convolutional network based on the U-net12 modified to include a dilated convolution block4 as well as dense13 and residual14 connections (Figure 2).The subfields were weighted within terms of their respective volumes in the training data set in order to account for the inter- and intra- class imbalance (Figure 3A). Dice Similarity Coefficient (DSC) and volume comparisons were used as evaluation metrics for the segmentations.
Results
Figure 4 visualizes a preprocessed T1w and T2w image and the corresponding manual and automated predicted segmentation. All subfields were segmented, and an overall mean DSC of 0.7723 was obtained. The segmentation of individual subfields achieves a mean DSC of ≥0.75 for ERC, Sub, CA1, and DG; and ≥0.58 for CA2, CA3, and Tail (Figure 3B). The average subfield volumes predicted are presented in Figure 5A and is compared to the manual segmentations by percentage deviation. Subfield ERC, CA2, CA3, and Tail were all oversegmented with a maximum deviation of 54.2 %, and Sub, CA1, and DG were undersegmented with a maximum deviation of 8.6 %.The network achieved an average inference time of 2 minutes and 16 seconds per hippocampi.Discussion
In contrast to other deep learning methods, which have aggregated small subfields4-6, this study segments smaller subfields individually. The proposed method segmented hippocampal subfields at a level comparable to ASHS9 and raters in UMC Utrecht9, but inferior to raters in Magdeburg10 (Figure 5B). A lower performance in DSC and volume comparison were observed for the smaller subfields, which is likely still caused by the class imbalance and suggests a need for better weighting. The volume comparison indicates that the weighting is biased in favor of smaller subfields, as smaller subfields are oversegmented and larger subfields undersegmented (ERC being an exception). Another optimization could be to include more data, specifically from the smaller subfields.Notably, the results in this work are achieved on a combined data set of two different scanners and MR protocols, indicating that the network can reliably handle images of different properties if included in training. The inclusion of multiple contrasts increases available information for the network, which should lead to a better segmentation. This was evident in early preliminary testing; however, the final efficacy of the multi-contrast approach has yet to be tested in this work. Inference time of the proposed method is vastly faster (> x100) than atlas based methods, such as ASHS (>24 hours) or FreeSurfer (~ 18 hours for whole MRI scan).9,15
Conclusion
Comparable results to earlier methods were achieved, using the proposed deep learning network, 7T MR images and the two contrasts T1w (MP-RAGE) and T2w (TSE). Inference time was faster than atlas-based methods for subfield segmentation, and the proposed method demonstrates robustness to scanner variety indicating clinical applicability. However, the results were obtained using 7T MR scanners, whereas 3T scanners are primarily used in clinical practice.16 Therefore, it could be relevant to apply this method on 3T MR images and test whether the performance would be affected due to the lower resolution.Acknowledgements
MB acknowledges funding from Australian Research Council Future Fellowship grant FT140100865. This research was undertaken with the assistance of resources and services from the Queensland Cyber Infrastructure Foundation (QCIF). DRL, MTG, and TJ acknowledge funding from the following Danish private foundations: Otto Mønsteds Foundation, Knud Højgaards Foundation, Oticon Foundation - William Demant Foundation, Familien Hede Nielsens Foundation, Marie and M.B Richters Foundation, Gerda Mørup-Petersens Familie- og Studielegat, Henry Shaws Legat, Sanatorielæge Ellen Pedersens Legat, Nordea Foundation, and Keflico Foundation. The authors acknowledge the facilities and scientific and technical assistance of the National Imaging Facility (NIF), a National Collaborative Research Infrastructure Strategy (NCRIS) capability, at the Centre for Advanced Imaging, The University of Queensland.References
1. Boutet C, Chupin M, Lehéricy S, et al. Detection of volume loss in hippocampal layers in Alzheimer's disease using 7 T MRI: A feasibility study. NeuroImage: Clinical. 2014;5:341-348.
2. La Joie R, Perrotin A, de La Sayette V, et al. Hippocampal subfield volumetry in mild cognitive impairment, Alzheimer's disease and semantic dementia. NeuroImage: Clinical. 2013;3:155-162.
3. Maruszak A, Thuret S. Why looking at the whole hippocampus is not enough - a critical role for anteroposterior axis, subfield and activation analyses to enhance predictive value of hippocampal changes for Alzheimer's disease diagnosis. Frontiers in Cellular Neuroscience. 2014;8.
4. Zhu H, Shi F, Wang L, et al. Dilated Dense U-Net for Infant Hippocampus Subfield Segmentation. Frontiers in Neuroinformatics. 2019;13.
5. Li G, Chen M, Li G, et al. A Preliminary Volumetric MRI Study of Amygdala and Hippocampal Subfields in Autism During Infancy. IEEE International Symposium on Biomedical Imaging. 2019:1052-1056.
6. Jiang H, Guo Y. Multi-class Multimodal semantic segmentation with an improved 3D Fully Convolutional Networks. Neurocomputing. 2019.
7. Wu Z, Gao Y, Shi F, et al. Segmenting Hippocampal Subfields from 3T MRI with Multi-modality Images. Medical Image Analysis. 2018;43:10-22.
8. ASHS: Automatic Segmentation of Hippocampal Subfields. NeuroImaging Tools \& Resources Collaboratory. March 22, 2019. https://www.nitrc.org/projects/ashs. Accessed October 4, 2019.
9. Wisse L, Kuijf H, Honingh A, et al. Automated Hippocampal Subfield Segmentation at 7T MRI. American Journal of Neuroradiology. 2016;37(6):1050-1057.
10. Berron D, Vieweg P, Hochkeppler A, et al. A protocol for manual segmentation of medial temporal lobe subregions in 7 Tesla MRI. NeuroImage: Clinical. 2017;15:466-482.
11. Gorgolewski K, Burns C, Madison C, et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in Python. Frontiers in Neuroinformatics. 2011;5(13).
12. Ronneberger O, Fischer P, Brox T. U-Net: Convolutional Networks for Biomedical Image Segmentation. Medical Image Computing and Computer-Assisted Intervention. 2015:234-241.
13. Huang G, Liu Z, van der Maaten L, et al. Densely Connected Convolutional Networks. IEEE Conference on Computer Vision and Pattern Recognition. 2017:2261-2269.
14. He K, Zhang X, Ren S, et al. Deep Residual Learning for Image Recognition. IEEE Conference on Computer Vision and Pattern Recognition. 2016:770-778.
15. Sone D, Sato N, Maikusa N, et al. Automated subfield volumetric analysis of hippocampus in temporal lobe epilepsy using high-resolution T2-weighed MR imaging. NeuroImage: Clinical. 2016;12:57-64.
16. van der Kolk A, Hendrikse J, Zwanenburg J, et al. Clinical applications of 7 T MRI in the brain. European Journal of Radiology. 2013;82(5):708-718.
Figures