3513
Automated Quantification of Lung Cysts at 0.55T MRI with Image Synthesis from CT using Deep Learning1National Institutes of Health, Bethesda, MD, United States
Synopsis
We propose a novel machine learning approach for segmentation of lung cystic structures using MRI. Following our recent development on improved structural lung imaging at low-field MRI we use a combination of generative adverserial networks and modified UNet for segmentation of cyst and lung tissues. This provides a non-ionizing radiation free alternative for patients with Lymphangioleiomyomatosis who are evaluated using CT imaging. We employ cross-modality image synthesis and segmentation approaches which work synergistically to take advantage of available CT data. In this work we demonstrate the potential of MRI for quantitative analysis of cystic lung .
INTRODUCTION
Lymphangioleiomyomatosis (LAM) [1] is a degenerative lung disease, affecting mostly women of childbearing age, leading to formation of cysts in the lung parenchyma. CT imaging is commonly used for repeated assessment of the cystic disease. The burden of cysts in the lungs is calculated by the “cyst score”, defined as the ratio of cyst volume to lung volume [2,3]. While lung imaging is difficult at 1.5 or 3T MR due to short T2* in lung, we have demonstrated that high-performance low field MRI provides high contrast images in the lung [4] and offers good delineation of cysts. This opens a new avenue for radiation-free lung cyst imaging. Analysis tools are lacking for lung MR images, while commercial software is available for CT cyst quantification. In addition, there are few MR data patient images available, compared to CT. We proposed a deep learning approach utilizing the rich CT datasets with image synthesis to train deep learning models for MR. We demonstrate automated detection of lung cysts and the calculation of cyst score for the first time with MRI.METHODS
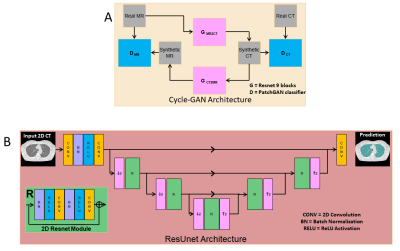
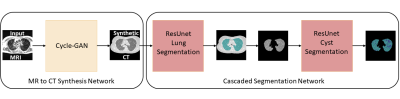
Deep Learning Pipeline: Figure (1A) shows the generative adversarial network used for unsupervised learning of MR to CT synthesis. We use the architecture of CycleGAN [5] to train, two 9-block Resnet generators to synthesize MRI to CT and vice-versa, and two PatchGAN [6] discriminators to distinguish between fake (synthesized) MRI and CT. For segmentation, we use a modified Residual UNet [7,8] with cascaded ResUNet architecture for sequential segmentation of lung volume and cysts. As shown in Figure (1B), skipped connections are introduced between downsampling and upsampling branches. The loss function used was a weighted sum of cross-entropy loss and Jaccard index [9], which jointly optimizes the classification accuracy and overlap of predicted mask and ground truth. Figure (2) shows the proposed pipeline. First, the MR image is converted to a synthesized CT image using a forward pass of the Cycle-GAN network. Then, the synthesized CT image is passed through the cascaded ResUnets for lung and cyst mask prediction.Data: 65 patients diagnosed with LAM were imaged using our high-performance 0.55T MRI scanner (prototype Aera, MAGNETOM, Siemens Healthcare, Erlangen, Germany) to generate T2-weighted images of the lung parenchyma (turbo spin echo sequence, TE/TR = 47/4403ms, FOV = 270mmx360mm, matrix = 480x640, 32 slices, slice thickness = 6mm, bandwidth = 260Hz/Px, respiratory triggered). Standard chest CT images (Aquilion One, Canon Medical, Japan) were acquired using 0.5mm x 80 detector rows, 120 or 100kV, Automatic Exposure control, 0.275s rotation speed and standard helical pitch. CT images were reconstructed to 2 mm slice thickness using lung kernel, 512x512 image matrix resulting in a spatial resolution of 0.8mmx0.8mm. In total, 14016 CT images and 1258 MR images were included in the data cohort.
Training: 55 patients were used for training and 5 for validation. 5 subjects were held out as test subjects to evaluate the proposed cyst score calculation. The Cycle-GAN network was trained using 12089 CT slices and 1258 MRI slices. The loss function used is the sum of two adversarial losses and the cycle-consistency loss. For training the ResUNets, ground truth was obtained using K-means clustering of the CT images. Obtaining manually annotated labels is infeasible due to dense cystic distribution and highly varying cyst sizes. All images were resized to a resolution of 1.5 mmx1.5 mm and matrix size of 256x256 and normalized to have zero mean and unit standard deviation.
RESULTS
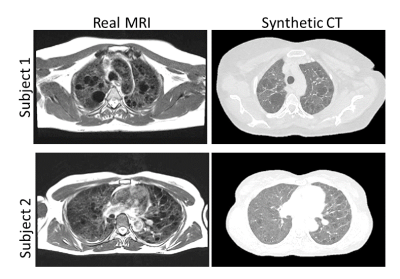
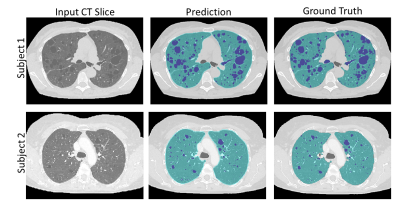
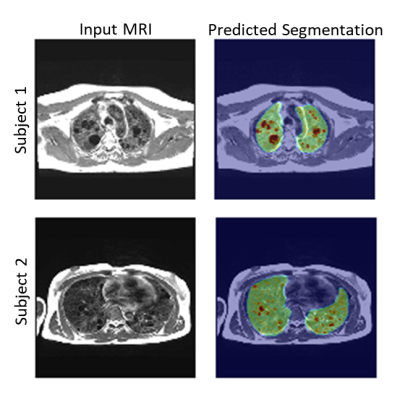
Figure (3) shows typical synthetic CT generated using our MRI to CT Cycle-GAN. They are visually similar to the real CT images and preserve considerable anatomical details. The trained classifier network successfully segmented the lungs and cysts in the real CT images (Figure (4)). The average dice sore across test subjects is 0.94 ± 0.1 and 0.86 ± 0.03 for the lung and cyst segmentation network respectively. Figure (5) shows the performance of the entire pipeline for MRI data segmentation. The segmentation of the synthetic CT using the cascaded ResUnet is overlaid on the real MR slice.DISCUSSION & CONCLUSION
In this study we proposed a deep learning approach to segment cysts using lung MRI, taking advantage of improved lung anatomical imaging provided by a high-performance 0.55T MRI system. An ionizing radiation-free alternative to quantify cyst score is beneficial for patients with LAM. Since less data is available from MR lung imaging, a CycleGAN based image synthesis strategy was developed to utilize rich CT data collection for MR processing. Initial validation showed lung volume and cysts can be well segmented using a cascaded neural networks. Future work will improve the network architecture to enforce shape integrity to avoid loss of anatomical details in the cross-domain synthesis step. Augmentation techniques could increase robustness of the model to classify smaller cysts and improving generalizability.Acknowledgements
Funding was provided by the National Heart, Lung, and Blood Institute’s Division of Intramural Research. We would like to acknowledge the assistance of Siemens Healthcare in the modification of the MRI system for operation at 0.55T under an existing cooperative research agreement (CRADA) between NHLBI and Siemens Healthcare.References
1. Taveira-Dasilva, Angelo M., Wendy K. Steagall, and Joel Moss. "Lymphangioleiomyomatosis." Cancer control 13.4 (2006): 276-285.
2. Schmithorst, Vincent J., et al. "Automated algorithm for quantifying the extent of cystic change on volumetric chest CT: initial results in Lymphangioleiomyomatosis." American Journal of Roentgenology 192.4 (2009): 1037-1044.
3. Yao, Jianhua, et al. "CT grading of lung disease in lymphangioleiomyomatosis." American Journal of Roentgenology 199.4 (2012): 787-793.
4. Campbell-Washburn, Adrienne E., et al. "Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI." Radiology (2019): 190452.
5. Jun-Yan Zhu*, Taesung Park*, Phillip Isola, and Alexei A. Efros. "Unpaired Image-to-Image Translation using Cycle-Consistent Adversarial Networks", in IEEE International Conference on Computer Vision (ICCV), 2017.
6. Isola, Phillip, et al. "Image-to-image translation with conditional adversarial networks." Proceedings of the IEEE conference on computer vision and pattern recognition. 2017.
7.Xue, Hui, et al. "Automated Detection of Left Ventricle in Arterial Input Function Images for Inline Perfusion Mapping using Deep Learning: A study of 15,000 Patients." arXiv preprint arXiv:1910.07122 (2019).
8. Ronneberger, Olaf, Philipp Fischer, and Thomas Brox. "U-net: Convolutional networks for biomedical image segmentation." International Conference on Medical image computing and computer-assisted intervention. Springer, Cham, 2015.
9. Shvets, Alexey A., et al. "Automatic instrument segmentation in robot-assisted surgery using deep learning." 2018 17th IEEE International Conference on Machine Learning and Applications (ICMLA). IEEE, 2018.
Figures