3498
Image Reconstruction with Low-rankness and Self-consistency of k-space Data in Parallel MRI1Department of Electronic Science, Fujian Provincial Key Laboratory of Plasma and Magnetic Resonance, Xiamen University, Xiamen, China, 2School of Computer and Information Engineering, Fujian Provincial University Key Laboratory of Internet of Things Application Technology, Xiamen University of Technology, Xiamen, China, 3Department of Computer Science, School of Information Science and Engineering, Xiamen University, Xiamen, China, 4Neusoft Medical System, Shanghai, China
Synopsis
Recent low-rank reconstruction methods offer encouraging image reconstruction results enabling promising acceleration of parallel magnetic resonance imaging, however, they were not originally designed to exploit the routinely acquired calibration data for performance improvement in parallel magnetic resonance imaging. In this work, we proposed an image reconstruction approach to simultaneously explore the low-rankness of the k-space data and mine the data correlation among multiple receiver coils with the use of the calibration data. The proposed method outperforms the state-of-the-art methods in terms of suppressing artifacts and achieving lowest error, and exhibits robust reconstructions even with limited auto-calibration signals.
Introduction
Prolonged acquisition time poses a challenging task in magnetic resonance imaging (MRI) 1. Parallel imaging technology significantly reduces the scan time, however, limited by the requirement of sufficient auto-calibration signal (ACS) 2-4. Sparse sampling with reconstruction is another effective way to speed up the data acquisition duration, and the reconstruction methods that could be categorized into two main classes: sparsity approach and low-rankness approaches. Rooting from low rankness methods, the recent emergence of low-rank structured matric methods, exhibit very promising results in MRI 5-8 and magnetic resonance spectroscopy 9-12. However, there are still limitations of the methods, for instance, separately enforcing the low rankness of horizontal and vertical directions results in suboptimal solution in ALOHA 6, existed low-rank structured matric methods did not take advantage of the common acquired ACS to help improve reconstruction performance. Here we proposed a k-space domain reconstruction method that concurrently explores the low-rankness of k-space data and calibration data. The low rankness was exploited by minimizing the low-rankness of the two-directional weighting of the k-space. In addition, intra- and inter-coil data relationships are enforced in the form of self-consistency over k-space.Methods
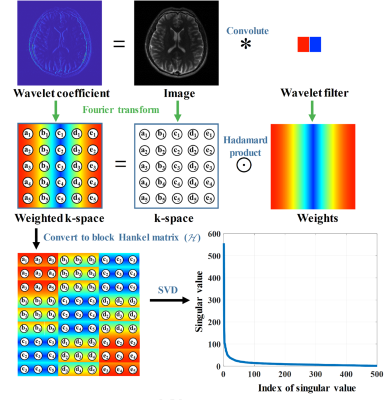
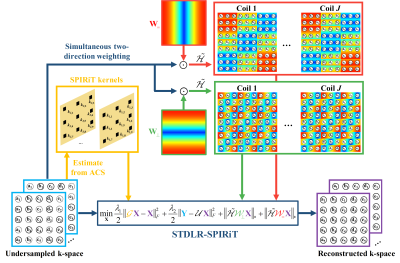
It has been proved that the sparsity of image has a close relationship to the low rankness of the k-space data (Fig. 1). The recovery of missing k-space data can be done through minimizing a matric completion problem with the enforcement of low rankness of the Hankel matric of weighting k-space data. We proposed a reconstruction method named STDLR-SPIRiT to exploit the simultaneous two-directional low rankness (STDLR) of k-space data and the information of ACS signal utilized with a SPIRiT term: $$\left( \mathbf{STDLR-SPIRiT} \right) \quad \underset{\mathbf{X}}{\mathop{\min }}\,\sum\limits_{i}{{{\left\| \tilde{\mathcal{H}}{{\mathcal{W}}_{i}}\mathbf{X} \right\|}_{*}}}+\frac{{{\lambda }_{1}}}{2}\left\| \mathcal{G}\mathbf{X}-\mathbf{X} \right\|_{F}^{2}+\frac{{{\lambda }_{2}}}{2}\left\| \mathbf{Y}-\mathcal{U}\mathbf{X} \right\|_{F}^{2},$$ where $$$i$$$ denotes $$$=$$$ or $$$\bot $$$, i.e. horizontal and vertical directions. $$$\mathbf{X}=\left[ {{\mathbf{X}}_{1}},{{\mathbf{X}}_{2}},\cdots ,{{\mathbf{X}}_{J}} \right]$$$ denotes the targeted k-space data from all coils and $$$\mathbf{Y}=\left[ {{\mathbf{Y}}_{1}},{{\mathbf{Y}}_{2}},\cdots ,{{\mathbf{Y}}_{J}} \right]$$$ the acquired k-space data with zero-filling at non-acquired positions, $$$\tilde{\mathcal{H}}{{\mathcal{W}}_{i}}\mathbf{X}=\left[ \mathcal{H}{{\mathbf{W}}_{i}}\odot {{\mathbf{X}}_{1}},\cdots ,\mathcal{H}{{\mathbf{W}}_{i}}\odot {{\mathbf{X}}_{J}} \right]$$$, $$$\mathcal{U}$$$represents the operator that performs undersampling and zerofilling on non- acquired data points, $$$\mathcal{H}$$$ is an operator that converts a matric into a block Hankel matrix and $$${{\mathbf{W}}_{i}}$$$ denote the weights which are the Fourier transform of filters in the horizontal or vertical directions, $$$\mathcal{G}$$$ is an operator thar convolves the k-space data with a series of calibration kernels that are estimated from ACS. $$${{\left\| \cdot \right\|}_{*}}$$$ imposes the low-rank constraint on the weighted Hankel structured matrix, $$${{\lambda }_{1}}$$$ and $$${{\lambda }_{2}}$$$ trade off among the low-rank constraint, self-calibration consistency and undersampled data fidelity. The second proposed approach uses the same calibration g as the SPIRiT. The schematic illustration of STDLR-SPIRiT is depicted in Fig. 2.We further proposed the SVD-free version of STDLR-SPIRiT to reduce the computational time $$\underset{\mathbf{X},{{\mathbf{P}}_{i}},{{\mathbf{Q}}_{i}}}{\mathop{\min }}\,\frac{1}{2}\sum\limits_{i}{\left( \left\| {{\mathbf{P}}_{i}} \right\|_{F}^{2}+\left\| {{\mathbf{Q}}_{i}} \right\|_{F}^{2} \right)}+\frac{{{\lambda }_{1}}}{2}\left\| \mathcal{G}\mathbf{X}-\mathbf{X} \right\|_{F}^{2}+\frac{{{\lambda }_{2}}}{2}\left\| \mathbf{Y}-\mathcal{U}\mathbf{X} \right\|_{F}^{2} \quad s.t. \quad {{\mathbf{P}}_{i}}\mathbf{Q}_{i}^{H}=\tilde{\mathcal{H}}{{\mathcal{W}}_{i}}\mathbf{X},$$ where $$${{\mathbf{P}}_{i}}$$$ and $$${{\mathbf{Q}}_{i}}$$$ denote factorized matrices, and the upper subscript $$$H$$$ denotes the Hermitian transpose of a complex matrix. Here, we adopt the alternating direction method of multiplier to deal with the SVD-free version proposed model.
Results
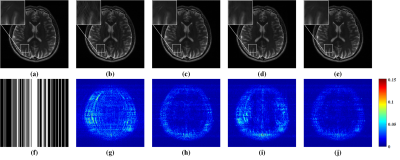
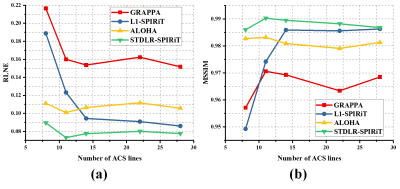
As shown in Fig. 3, GRAPPA 3 and ALOHA 6 produce image with obvious artifacts while $$$\ell_1$$$-SPIRiT 4 and STDLR-SPIRiT provide much better reconstructed image with good artifacts suppression. But, when inspecting on zoom-in images, we can find relatively stronger noise and somewhat blur of details in the $$$\ell_1$$$-SPIRiT reconstruct image. The proposed STDLR-SPIRiT reconstructs an image with better signal to noise ratio and fine details preservation. Moreover, as shown in Fig. 4, STDLR-SPIRiT shows robustness to the number of ACS lines.Conclusion
We present a parallel imaging reconstruction method called STDLR-SPIRiT to simultaneously utilize the self-consistency of k-space data and the low-rankness of the weighted k-space data. The proposed method allows superior performance in artifacts suppression and edge preservation than the state-of-the-art methods. More importantly, the proposed approach does not be sensitive to calibration data.Acknowledgements
This work was supported in part by National Key R&D Program of China (2017YFC0108703), National Natural Science Foundation of China (61971361, 61571380, 61871341, 61811530021, U1632274, 61672335 and 61671399), Natural Science Foundation of Fujian Province of China (2018J06018), Fundamental Research Funds for the Central Universities (20720180056), Science and Technology Program of Xiamen (3502Z20183053), and China Scholarship Council.
The correspondence should be sent to Dr. Xiaobo Qu (Email: quxiaobo@xmu.edu.cn).
References
[1] J. Hamilton, D. Franson, and N. Seiberlich, “Recent advances in parallel imaging for MRI,” Progress in Nuclear Magnetic Resonance Spectroscopy, vol. 101, pp. 71–95, 2017.
[2] K. P. Pruessmann, M. Weiger, M. B. Scheidegger, and P. Boesiger, “SENSE: Sensitivity encoding for fast MRI,” Magnetic Resonance in Medicine, vol. 42, pp. 952–962, 1999.
[3] M. A. Griswold, P. M. Jakob, R. M. Heidemann, M. Nittka, V. Jellus, J. Wang, B. Kiefer, and A. Haase, “Generalized autocalibrating partially parallel acquisitions (GRAPPA),” Magnetic Resonance in Medicine, vol. 47, no. 6, pp. 1202–1210, 2002.
[4] M. Lustig and J. M. Pauly, "SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space," Magnetic Resonance in Medicine, vol. 64, no. 2, pp. 457-71, 2010.
[5] P.J.Shin, P.E.Z.Larson, M.A.Ohliger, M.Elad, J.M.Pauly, D.B.Vigneron, and M. Lustig, “Calibrationless parallel imaging reconstruction based on structured low-rank matrix completion,” Magnetic Resonance in Medicine, vol. 72, no. 4, pp. 959–970, 2014.
[6] K. H. Jin, D. Lee, and J. C. Ye, “A general framework for compressed sensing and parallel mri using annihilating filter based low-rank hankel matrix,” IEEE Transactions on Computational Imaging, vol. 2, no. 4, pp. 480–495, 2016.
[7] J. P. Haldar, "Low-rank modeling of local k-space neighborhoods (LORAKS) for constrained MRI," IEEE Transaction on Medical Imaging, vol. 33, no. 3, pp. 668-81, 2014.
[8] G. Ongie and M. Jacob, "Off-the-grid recovery of piecewise constant images from few Fourier samples," SIAM Journal on Imaging Sciences, vol. 9, no. 3, pp. 1004-1041, 2016.
[9] X. Qu, M. Mayzel, J.-F. Cai, Z. Chen, and V. Orekhov, "Accelerated NMR spectroscopy with low-rank reconstruction," Angewandte Chemie International Edition, vol. 54, no. 3, pp. 852-854, 2015.
[10] J. Ying, H. Lu, Q. Wei, J.-F. Cai, D. Guo, J. Wu, Z. Chen, and X. Qu, "Hankel matrix nuclear norm regularized tensor completion for N-dimensional exponential signals," IEEE Transactions on Signal Processing, vol. 65, no. 14, pp. 3702-3717, 2017.
[11] J. Ying, J.-F. Cai, D. Guo, G. Tang, Z. Chen, and X. Qu, "Vandermonde factorization of Hankel matrix for complex exponential signal recovery-application in fast NMR spectroscopy," IEEE Transactions on Signal Processing, vol. 66, no. 21, pp. 5520-5533, 2018.
[12] H. Lu, X. Zhang, T. Qiu, J. Yang, J. Ying, D. Guo, Z. Chen, and X. Qu, "Low rank enhanced matrix recovery of hybrid time and frequency data in fast magnetic resonance spectroscopy," IEEE Transactions on Biomedical Engineering, vol. 65, no. 4, pp. 809-820, 2018.
Figures