3452
Highly accelerated T1ρ imaging with Kernel-based low-rank compressed sensing: evaluation of retrospective and prospective undersampling1Biomedical Engineering, Cleveland Clinic, Cleveland, OH, United States, 2Program of Advanced Musculoskeletal Imaging (PAMI), Cleveland Clinic, Cleveland, OH, United States, 3Electrical Engineering, University at Buffalo, the State University of New York, Buffalo, NY, United States, 4Biomedical Engineering, University at Buffalo, the State University of New York, Buffalo, NY, United States, 5Department of Diagnostic Radiology, Cleveland Clinic, Cleveland, OH, United States
Synopsis
Quantitative T1ρ MRI provides valuable information on compositional changes in cartilage, but requires longer scan time compared to conventional imaging. In this work, kernel-based low-rank compressed sensing reconstruction was used to accelerate T1ρ imaging and the retrospective and prospective undersampled results from four subjects were compared to reference T1ρ values.
Purpose
Quantitative MRI provides valuable information on compositional changes in soft tissues. Among the techniques, numerous studies have suggested that T1ρ imaging could be used to detect early cartilage degeneration in osteoarthritis.(1, 2) However, the long scan time of T1ρ due to the necessity of acquiring multiple images remains as a challenge for clinical application. Accelerated T1ρ imaging combining parallel imaging and compressed sensing have shown promising results.(3, 4) However, previous studies primarily applied retrospective downsampling and no studies yet have reported results from prospective downsampling. In this work, we applied a novel Kernel-based low-rank (KLR) compressed sensing reconstruction and demonstrated the feasibility of accelerated T1ρ imaging with retrospective and prospective downsampling in the knee.Methods
In the quantitative T1ρ technique, the exponential decay model represents the underlying relationship between echo images. However, additional factors in actual data acquisition such as noise and motion effect further complicate this relationship. To solve this, we used the kernel-based low-rank (KLR) method to reconstruct the images. Specifically, temporal bases were learned from low-resolution images obtained from a fully sampled center k-space of each echo. These temporal bases can represent not only the exponential model but also the noise and motion effects. Then an optimization problem was formulated by adding temporal bases as constraint along with a data fidelity term, which can be solved by an iterative algorithm. The details of the KLR method can be found in (5).Four volunteers were studied using a 3T MR scanner (Magnetom Prisma, Siemens Healthcare AG, Erlangen, Germany) with a 1Tx/15Rx knee coil (QED). The imaging protocol included 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient-echo snapshots (MAPSS) T1ρ imaging (6, 7) and dual-echo steady-state (DESS) imaging sequences. For retrospective undersampling, two healthy volunteers were scanned with parallel imaging (GRAPPA) factor of 2, and reconstructed K-space data was used to simulate retrospective undersampling. Undersampling factors (denoted as RF) of 4, 6, and 8 were simulated. For prospective undersampling, the mask generated during retrospective undersampling simulation was implemented to the sequence program. Two healthy subjects were scanned with the implemented sequence with RF of 4, 6, and 8, respectively. For both retrospective and prospective cases, imaging with 2x GRAPPA was used as reference image. Detailed sequence parameters are listed at Table 1. For KLR compressed sensing reconstruction, the number of principal components was set to 6, and the soft threshold value of 10 was used.
For T1ρ quantification, a two-parameter mono-exponential fitting was performed. Automatic segmentation was performed on the first echo of T1ρ image to segment six compartments (medial/lateral femur [MFC/LFC], medial/lateral tibia [MT/LT], trochlea [TRO], and patellar [PAT]). For prospective undersampling, a deep learning automatic segmentation was used on the DESS image to segment four compartments (Femur [FC], medial/lateral tibia [MT/LT], and patellar [PAT]). The coefficient of variations (CVs) were calculated between the reference and different undersampled results.
Results
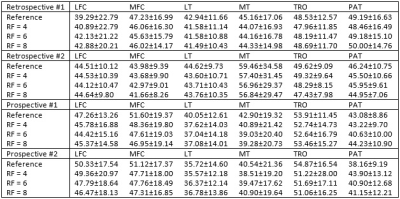
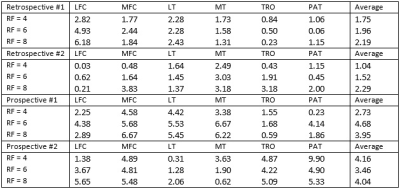
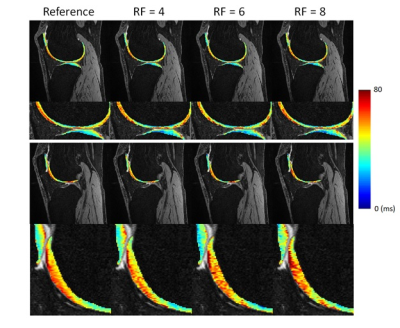
Table 2 summarized the quantitative results for the T1ρ map generated from the compressed-sensing reconstruction of both retrospectively and prospectively undersampled acquisition. For retrospective undersampling, the average CVs with respect to the reference value increased with higher RFs, but the overall average was lower than 3% (Table 3). With prospective undersampling, the overall CV increased compared to the retrospectively undersampled reconstruction, but the average CVs stayed under 5%. Figure 1 shows two slices of T1ρ maps from prospective undersampling.Discussion
With our novel compressed sensing reconstruction, 8 echoes of T1ρ weighted images could be collected within 4 minutes to generate a T1ρ map within 5% CV of the reference T1ρ map. The CVs were comparable to the scan-rescan repeatability of in vivo T1ρ imaging reported in the literature.(2) 3D MAPSS T1ρ imaging with RF cycling and variable flip angles has been validated to provide more accurate and reliable T1ρ quantification by minimizing the filter effect in k-space and T1 recovery contamination (6, 7). However, RF cycling requires a double scan time. The proposed acceleration method in the study will significantly reduce the acquisition time while keeping the quantification accuracy. In this study, the reconstruction based on the KLR algorithm showed promising results. No spatial regularization was applied within each echo images. We will investigate if combining spatial regularization and KLR will further improve quantification accuracy. There also remains a question regarding the discrepancy between retrospective simulation and actual prospectively undersampled outcome, which can be contributed by the different order of k-space data acquisition and different noise distribution between the simulation and actual acquisition. Different schemes of acquisition ordering will be implemented and evaluated in the future.Conclusion
In this study, we have demonstrated the feasibility of highly accelerated T1ρ imaging with high quantification accuracy by using prospective downsampling and novel compressed sensing reconstruction. To confirm the results warrants large scale studies. Such novel acceleration techniques will significantly facilitate the rapid clinical translation of quantitative MRI techniques.Acknowledgements
No acknowledgement found.References
1. Atkinson HF, Birmingham TB, Moyer RF, Yacoub D, Kanko LE, Bryant DM, et al. MRI T2 and T1rho relaxation in patients at risk for knee osteoarthritis: a systematic review and meta-analysis. BMC musculoskeletal disorders. 2019;20(1):182
2. MacKay JW, Low SBL, Smith TO, Toms AP, McCaskie AW, Gilbert FJ. Systematic review and meta-analysis of the reliability and discriminative validity of cartilage compositional MRI in knee osteoarthritis. Osteoarthritis and cartilage. 2018;26(9):1140-52
3. Zhou Y, Pandit P, Pedoia V, Rivoire J, Wang Y, Liang D, et al. Accelerating T1rho cartilage imaging using compressed sensing with iterative locally adapted support detection and JSENSE. Magnetic resonance in medicine. 2016;75(4):1617-29.
4. Zibetti MVW, Sharafi A, Otazo R, Regatte RR. Accelerating 3D-T1rho mapping of cartilage using compressed sensing with different sparse and low rank models. Magnetic resonance in medicine. 2018;80(4):1475-91.
5. Nakarmi U, Wang Y, Lyu J, Liang D, Ying L. A Kernel-Based Low-Rank (KLR) Model for Low-Dimensional Manifold Recovery in Highly Accelerated Dynamic MRI. IEEE transactions on medical imaging. 2017;36(11):2297-307
6. Li X, Han ET, Busse RF, Majumdar S. In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS). Magnetic resonance in medicine. 2008;59(2):298-307.
7. Li X, Pedoia V, Kumar D, Rivoire J, Wyatt C, Lansdown D, et al. Cartilage T1rho and T2 relaxation times: longitudinal reproducibility and variations using different coils, MR systems and sites. Osteoarthritis and cartilage. 2015;23(12):2214-23.
Figures